Scroll to:
Does coronary artery disease go hand-in-hand with bradyarrhythmias? An observational study from Eastern India
https://doi.org/10.15829/1560-4071-2025-5949
EDN: PEYNTC
Abstract
Aim. Many patients with conduction system disorders have associated heart conditions, especially ischemic heart disease. This study was designed to assess the prevalence and pattern of coronary artery disease (CAD) in patients with bradyarrhythmias requiring permanent pacemaker and association with CAD risk factors.
Material and methods. This single-centre observational cohort study included 80 patients with mean age of 63±9.4 years, admitted with symptomatic bradyarrhythmias. Patients underwent coronary angiography after obtaining informed consent and prevalence and pattern of CAD were analyzed in them. CAD was defined as narrowing in major epicardial coronary arteries or their first order branches and obstructive CAD as ≥50% stenosis. CAD was further categorized as single vessel disease, double vessel disease and triple vessel disease and any association with conduction system disorders studied.
Results. CAD was present in 56% patients and obstructive CAD in 37.5% patients. 19% patients had single vessel disease, while 18% had multi-vessel CAD. Obstructive LAD disease was seen in 25% of patients, followed by right coronary artery in 21.3% patients and LCX in 15% of patients. Heart team advised revascularization in majority (75%) of patients with obstructive CAD. AV nodal artery disease was found more in patients of complete heart block (p=0.0359). Among various risk-factors, dyslipidemia (56.7% vs 22%, p=0.0016), family history of CAD (63.3% vs 18%, p<0.0001) and angina (53.3% vs 20%, p=0.0020) showed significant association with obstructive CAD. RWMA on echocardiography (50% vs 14%, p=0.0004) and lower mean left ventricular ejection fraction (52.7% vs 58.1%, p=0.0270) also showed significant association with obstructive CAD.
Conclusion. In patients requiring permanent pacemaker, coexistent obstructive CAD was noted in 37.5% of the subjects. Causal association between obstructive CAD and conduction disturbances may not be established from this data, but the increased prevalence of atherosclerotic cardiovascular disease in patients having bradyarrhythmias implies the need for larger multi-center trials to understand the causal association and plan for earlier management.
For citations:
Wasil A.B., Kuila M., Mandal M., khanra D., Sharma R.K., Mukherjee A. Does coronary artery disease go hand-in-hand with bradyarrhythmias? An observational study from Eastern India. Russian Journal of Cardiology. 2025;30(2):5949. https://doi.org/10.15829/1560-4071-2025-5949. EDN: PEYNTC
Introduction
Bradyarrhythmias are defined as heart rate of less than 50 beats per minute and are common in clinical practice [1]. Various rhythm disturbances like sinus node dysfunction and atrioventricular conduction disturbances are included in bradyarrhythmias. They may occur normally as in well trained athletes (physiological) or may be pathological, and may be transient or permanent. Clinical presentation varies from typical symptoms like near syncope or syncope to atypical symptoms which include angina, dizziness, lethargy, confusional state, fatigue and dyspnea or the patient may have asymptomatic electrocardiography (ECG) changes [2]. Coronary artery disease (CAD) is the occurrence of an atherosclerotic plaque in the coronary arteries, which can be obstructive or non-obstructive. CAD may remain stable in a patient or sometimes can become unstable due to different types of acute thrombotic events [3].
Conduction system disorders are common in India with eastern India bearing a major brunt of the disease [4]. Patients undergoing cardiac Implantable electronic device implants are almost a decade younger in India compared to western world [5]. In addition, CAD occurs earlier in Indian population (5-10 years earlier compared to other populations) and the progression may also be faster [6]. Prevalence of CAD in patients with conduction system disorders has been reported to be 15-80% in different studies [7-9]. Presence of CAD can make the prognosis of patients with conduction disorders worse in addition to having a potential causative role. Limited data of such nature is available among Indian population and further less available from eastern India where the prevalence of the disease is among the highest [4]. This study was designed to assess the prevalence and pattern of CAD in patients with bradyarrhythmias requiring permanent pacemaker and association with CAD risk factors, in eastern Indian population.
Material and Methods
Study sample. This single-centre observational cohort study was conducted at our hospital from April 2020 to September 2021. Out of total 116 patients who needed permanent pacemaker implantation (PPI), 80 patients were included in the study who fulfilled the eligibility criteria, after obtaining an informed written consent (Figure 1). The study was approved by Institutional ethics committee (No. 1381).
Inclusion criteria. Patients who were ≥40 years of age and presented with symptomatic sinus node dysfunction, symptomatic sinus bradycardia or atrioventricular (AV) block due to a necessary drug with no alternative, symptomatic tachy-brady syndrome, chronotropic incompetence, acquired second-degree Mobitz type II AV block, high-grade AV block, or third-degree AV block, marked first-degree or second-degree Mobitz type I (Wenckebach) AV block with symptoms clearly attributable to the block, alternating bundle branch block and patients with syncope and bundle branch block with an His-ventricular interval of 70 ms or greater or evidence of infranodal block at electrophysiology study [2]. In addition, patients who had one or more conventional atherosclerotic cardiovascular disease risk-factors including smoking, hypertension, type 2 diabetes mellitus, dyslipidemia, obesity, family history of CAD or history of angina, were selected to undergo coronary angiography (CAG).
Exclusion Criteria. Patients with potentially reversible causes of bradyarrhythmia like acute coronary syndrome, myocarditis, infective endocarditis, dyselectrolemia especially hyperkalemia, or intake of drugs like digoxin, beta-blockers, calcium channel blockers or anti-arrhythmic medications were excluded from the study. In addition, patients who had other heart conditions like valvular heart diseases, congenital heart diseases, infiltrative disorders, connective tissue diseases or muscle dystrophies were excluded. Also patients with systemic malignancies and allergy to contrast agents or patients who refused to give consent were also excluded.
Study procedure. All the patients underwent clinical evaluation including detailed clinical history of present symptoms, past history, family history and history of CAD risk factors. Clinical examination was performed for presence of any signs of cardiovascular disorders or other relevant systemic signs. Bradyarrhythmias were diagnosed on the basis of 12 lead electrocardiogram or ECG evidence on Holter monitoring. Baseline investigations performed included Serum electrolytes, thyroid profile, kidney function tests, liver function tests, complete hemogram, fasting blood glucose, lipid-profile and serum uric acid to exclude other associated abnormalities. All patients underwent echocardiography for assessment of their left ventricular function, presence of other heart conditions like cardiomyopathies or structural heart diseases and evidence of wall motion abnormalities. All patients underwent CAG and permanent pacemaker was implanted as per the indication.
Coronary angiography. During index hospitalization, all the selected patients underwent CAG via trans-radial approach and the angiogram was assessed by two independent cardiologists. In most of the patients, 3 to 4 views were recorded for left coronary system and 2 for right coronary artery. All major coronary arteries and their first order branches were assessed for presence of stenosis and quality of blood flow in them. Reduction in luminal diameter of the diseased segment in comparison to disease free reference arterial segment was assessed visually and it determined the severity and percentage of CAD, which was defined as narrowing in major epicardial coronary arteries or their first order branches. A stenosis severity of ≥50% was defined as obstructive CAD. CAD was further categorized as single vessel disease, double vessel disease and triple vessel disease and any association with conduction system disorders was studied. Sinoatrial nodal artery (SNA) and atrioventricular nodal artery (AVNA) were identified and obstructive CAD in them was defined as ≥50% stenosis in the nodal artery or their feeding epicardial artery, proximal to the origin of nodal artery.
Statistical Analysis. Microsoft excel spreadsheet was used for data entry and then data was analyzed using SPSS (version 27.0; SPSS Inc., USA). Mean and standard deviation was derived for assessment of numerical variables and count and percentages for categorical variables. For comparison of groups, Student t test was used and for categorical variables, chi square test or fisher exact test was used as appropriate. A p value of <0.05 was considered statistically significant.
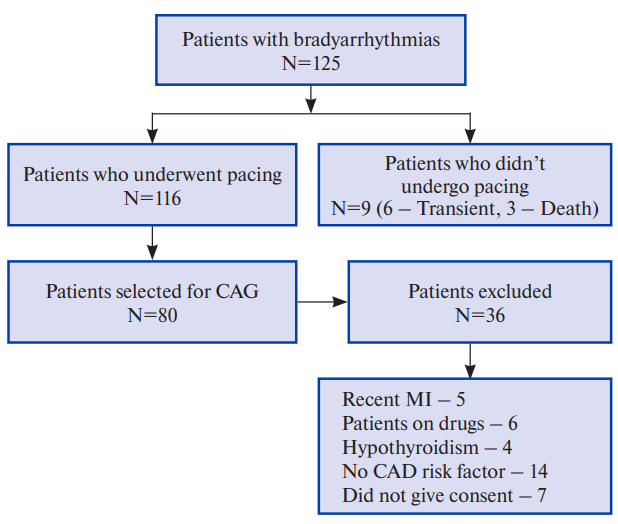
Figure 1. Consort flow chart of study schematic.
Abbreviations: CAD — coronary artery disease, CAG — coronary angiography, MI — myocardial infarction.
Results
Baseline patient characteristics
A total of 80 patients were enrolled in this study who presented to our department with symptomatic bradyarrhythmias for which permanent cardiac pacemaker was indicated. The study population comprised of 65% males and 35% females with a mean age of 63±9.4 years (age range of 40-81 years). Complete heart block (CHB) was present in 69% patients, followed by sick sinus syndrome (SSS) in 14% patients, bifascicular block in 9%, second degree AV block in 5% and trifascicular block in 4% patients.
CAG results
Out of 80 patients who underwent CAG, 44% patients had normal coronary arteries, 19% patients had non-obstructive CAD and 37.5% patients had obstructive CAD. With regard to number of vessels involved, single vessel disease was found in 19% patients, double vessel disease in 12% patients and triple vessel disease in 6% patients. Significant left anterior descending disease was seen in 25% patients, left circumflex disease was seen in 15% patients and right CAD was seen 21.3% patients. SNA disease was seen in 8.8% patients and AVNA disease was seen in 32.5% patients.
CAD and bradyarrhythmias
Among patients with obstructive CAD, 6.7% patients had bifascicular block, 76.7% patients had CHB, 6.7% patients had second degree AV block and 10.0% patients had SSS (Figure 2, Supplementary Table 1). No significant association was found between type of bradyarrhythmia and obstructive CAD. Although significant CAD was higher in patients with CHB, the association was not statistically significant (p=0.5196). Pattern of epicardial coronary vessel involvement also did not show significant association with indication for pacing. SNA disease was seen in 7.3% patients with CHB and 27.3% patients with SSS but the association was not statistically significant (p value =0.1833). AVNA disease was seen in 43.6% patients with CHB and the association was found to be statistically significant (p value =0.0359) (Figures 3, 4, 5, Supplementary Table 2).
Clinical variables and their relationship with CAG findings
For evaluation and correlation of significant CAD with different clinical variables and risk factors, the patients were divided into two groups. We found that presence of obstructive CAD was significantly associated with dyslipidemia (p value =0.0016), family history of CAD (p value <0.0001), history of angina (p value =0.0020), presence of regional wall motion abnormality (RWMA) on echocardiography (p value =0.0004) and lower mean left ventricular ejection fraction (p value =0.0270). No significant correlation was found between obstructive CAD and age (p value =0.2500), sex (p value =0.4676), presence of hypertension (p value =0.2261), type 2 diabetes mellitus (p value =0.0524), or history of smoking (p value =0.8589) as depicted in Table 1.
Table 1
Clinical variables and their relation with CAD
Variables | Significant CAD Absent N=50 (62.7%) | Significant CAD Present N=30 (37.5%) | P Value |
Mean Age (years) | 62.1±10.1 | 64.6±7.7 | 0.2500 |
Sex | |||
Male | 31 (62%) | 21 (70%) | 0.4676 |
Female | 19 (38%) | 09 (30%) | |
Hypertension | 35 (70%) | 17 (56.7%) | 0.2261 |
Type 2 Diabetes Mellitus | 10 (20%) | 12 (40%) | 0.0524 |
Smoking/Ex-Smoker | 19 (38%) | 12 (40%) | 0.8589 |
Angina | 10 (20%) | 16 (53.3%) | 0.0020 |
Dyslipidemia | 11 (22%) | 17 (56.7%) | 0.0016 |
Family history of CAD | 9 (18%) | 19 (63.3%) | <0.0001 |
Anemia | 22 (44%) | 18 (60%) | 0.1658 |
RWMA on Echo. | 7 (14%) | 15 (50%) | 0.0004 |
Mean LVEF (%) | 58.1±10.3 | 52.7±10.5 | 0.0270 |
Note: N — Number, % — Percentage.
Abbreviations: CAD — coronary artery disease, LVEF — left ventricular ejection fraction, RWMA — regional wall motion abnormality.
Supplementary Table 1
CAD in various types of bradyarrhythmias
Indication for Pacing | Significant CAD not present | Significant CAD present | Total |
Bifascicular block | 5 | 2 | 7 |
CHB | 32 | 23 | 55 |
Second degree AV block | 2 | 2 | 4 |
SSS | 8 | 3 | 11 |
Trifascicular block | 3 | 0 | 3 |
Total | 50 | 30 | 80 |
Note: Chi-square value: 3.2332; p-value: 0.5196.
Abbreviations: AV — atrioventricular, CAD — coronary artery disease, CHB — complete heart block, SSS — sick sinus syndrome.
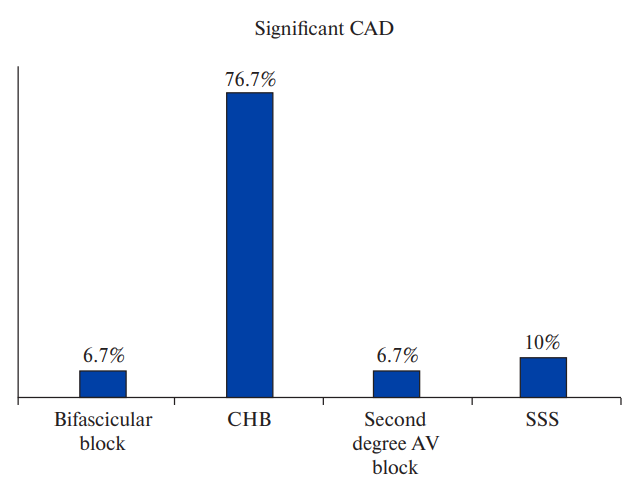
Figure 2a. Prevalence of significant CAD in different types of bradyarrhythmias.
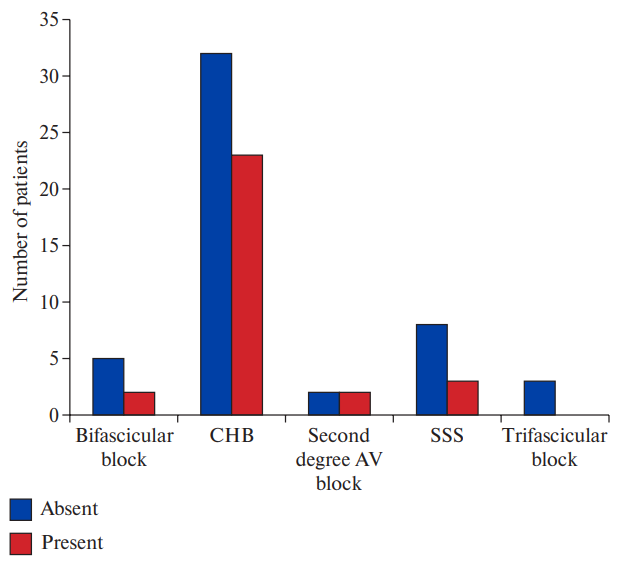
Figure 2b. Comparison between groups with and without CAD (Chi-square value: 3.2332; p-value: 0.5196).
Abbreviations: AV — atrioventricular, CAD — coronary artery disease, CHB — complete heart block, SSS — sick sinus syndrome.
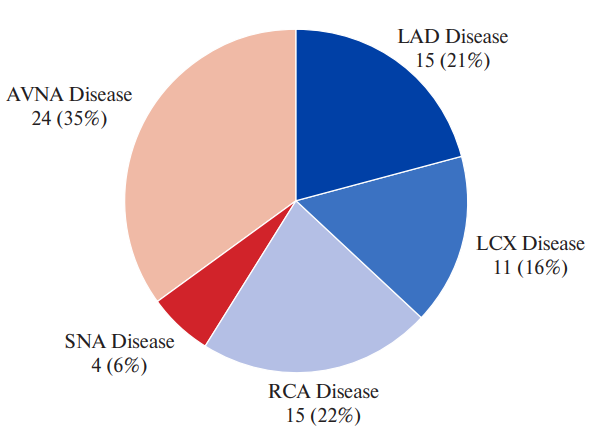
Figure 3. Various territories of CAD in patients with CHB.
Abbreviations: AVNA — atrioventricular nodal artery, LAD — left anterior descending, LCX — left circumflex, RCA — right coronary artery, SNA — sinoatrial nodal artery.
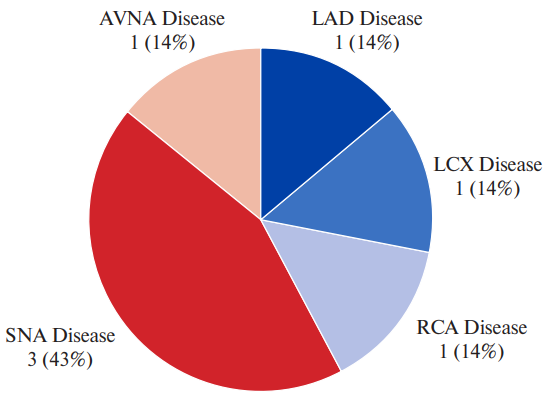
Figure 4. Various territories of CAD in patients with SSS.
Abbreviations: AVNA — atrioventricular nodal artery, LAD — left anterior descending, LCX — left circumflex, RCA — right coronary artery, SNA — sinoatrial nodal artery.
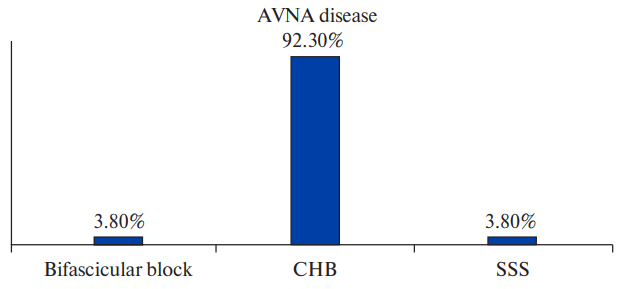
Figure 5a. AVNA disease in different types of bradyarrhythmias.
Abbreviations: AVNA — atrioventricular nodal artery, CHB — complete heart block, SSS — sick sinus syndrome.
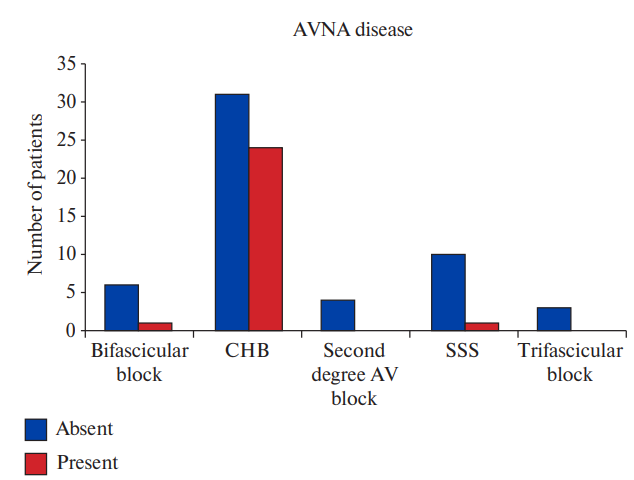
Figure 5b. Comparison between groups with and without AVNA disease in different types of conduction disorders (Chi-square value: 10.2860; p-value: 0.0359).
Abbreviations: AV — atrioventricular, AVNA — atrioventricular nodal artery, CHB — complete heart block, SSS — sick sinus syndrome.
Table 2
Various studies which have studied coexistent CAD in patients with bradyarrhythmias
Study | Number (N) | Male/Female | Mean age (years) | Prevalence of coexistent CAD (%) | Pattern | CAD pattern as per Mosseri classification | Artery involvement | Significant risk factors |
Mosseri M, et al. (1997) [8] | 43 | 32 (74.4%)/11 (25.6%) | 61±13 | 62.8 | Type I — 44.4%, Type II — 8.3%, Type III — 2.8%, Type IV — 44.4% | LAD — 46.5%, RCA — 41.8% | ||
Hsueh CW, et al. (2001) [15] | 113 | 68 (60.2%)/45 (39.8%) | 70.4±8 | 20 | LAD — 74%, LCX — 39.5%, RCA — 25.5% | Hypercholesterolemia, Diabetes Mellitus | ||
Brueck M, et al. (2008) [16] | 212 | 141 (66.5%)/71 (33.5%) | 70±9 | 71 | SVD — 15.1%, DVD — 17.5%, TVD — 38.2% | RCA — 58%, LAD — 54%, LCX — 52% | ||
Yesil M, et al. (2008) [17] | 203 | 121 (59.6%)/82 (40.4%) | 65±10 | 30.5 | Type I — 9.6%, Type II — 38.7%, Type III — 16%, Type IV — 35% | LAD — 28.1%, RCA — 15.7% | ||
Wei S, | 107 | 69 (64.5%)/38 (35.5%) | 63±9.6 | 74.7 | Type I — 7.5%, Type II — 26.3%, Type III — 15%, Type IV — 51.3% | RCA — 62.6%, LAD — 60.7% | ||
Alai MS, et al. (2016) [7] | 100 | 53 (53%)/47 (47%) | 64.6±10.7 | 29 | SVD — 24%, DVD — 7%, TVD — 14% | Type I — 6.9%, | Dyslipidemia, Smoking, Family history of CAD | |
Tayyebi M, et al. (2019) [19] | 102 | 53 (52%)/49 (48%) | 71.2±11.3 | 13.7 | LMCA — 28.6%, LAD — 28.6%, RCA — 28.6% | Male sex, Smoking, Angina, Diabetes Mellitus | ||
Vyas P, | 929 | 503 (54%)/426 (46%) | 67.8±24.7 | 34.4 | SVD — 15.3%, DVD — 10.2%, TVD — 8.9% | Type I — 5%, | Age ≥50 years, Male sex, Diabetes Mellitus, Hypertension | |
Kumar D, et al. (2024) [10] | 699 | 490 (70%)/209 (30%) | 66.75±9.37 | 12.45 | SVD — 6.15%, DVD — 3.6%, TVD — 2.7% | LAD — 7.9%, LCX — 1.0%, RCA — 3.6% |
Abbreviations: CAD — coronary artery disease, DVD — double vessel disease, LAD — left anterior descending, LCX — left circumflex, LMCA — left main coronary artery, RCA — right coronary artery, SVD — single vessel disease, TVD — triple vessel disease.
Supplementary table 2
Pattern of coronary artery involvement in different types of bradyarrhythmias
Variable | Bifascicular block | CHB | Second degree AV block | SSS | P value |
LAD disease present | 2 (10%) | 15 (75%) | 2 (10%) | 1 (5%) | 0.4037 |
LCX disease present | 0 | 11 (91.7%) | 0 | 1 (8.3%) | 0.4266 |
RCA disease present | 1 (5.9%) | 15 (88.2%) | 0 | 1 (5.9%) | 0.3725 |
SNA disease present | 0 | 4 (57.1) | 0 | 3 (42.9) | 0.1833 |
AVNA disease present | 1 (3.8%) | 24 (92.3%) | 0 | 1 (3.8%) | 0.0359 |
Abbreviations: AV — atrioventricular, AVNA — atrioventricular nodal artery, CAD — coronary artery disease, CHB — complete heart block, LAD — left anterior descending, LCX — left circumflex, RCA — right coronary artery, SNA — sinoatrial nodal artery, SSS — sick sinus syndrome.
Discussion
The cause of chronic bradyarrhythmias is not known in majority of the patients. It is difficult to ascribe a single cause, although fibrosis and degenerative calcification of the conduction system (Lenègre's disease, Lev's disease) has been implicated in many patients. Molecular or histological changes in conduction system may not completely explain the origin and genesis of conduction disorders. Other disease conditions like ischemic heart disease, may have some role to play in the pathophysiology of these disorders by causing degeneration of the conduction system due to long term ischemic damage, but it is difficult to establish a causal association [10]. These patients may evade the symptoms of ischemia due to lower heart rates, decreasing their cardiac energy needs, due to which their ischemic heart disease may remain undiagnosed or underdiagnosed, leading to poor clinical outcome in the long term. Various diagnostic modalities can be utilized for evaluation of CAD in these patients like dobutamine stress echocardiography, single-photon emission computed tomography, computed tomography-coronary angiography but invasive CAG remains the gold standard for conforming the diagnosis with approximately 0.1% major complication rate [11].
Most common indication for PPI in our study population was CHB (69%) followed by SSS (14%). Incidence of CAD in patients with chronic bradyarrhythmias have varied in different studies, ranging from 15-80% [7-9]. Among our study population, CAD was seen in 56% of patients and obstructive CAD in 37.5% of patients. The higher prevalence of CAD in our study group compared to similar studies from India may be attributed to higher prevalence of CAD in eastern India [12]. CAD pattern did not show any significant correlation with conduction disorders in contrast with Tandoğan I, et al. who found significant association between first perforator lesions or its proximal feeder vessel and right coronary artery lesions with requirement for PPI [13]. In our study, SNA disease was seen in 8.8% patients and AV Nodal artery disease was seen in 32.5% patients. And the association of AV nodal artery disease with CHB was statistically significant (p=0.0359). However, it needs to be remembered that SNA and AVNA are very small arteries and the flow quality of dye during CAG in them might not be good. In addition, any significant preceding lesion in the parent artery might lead to decreased or altered flow in these vessels. Table 2 compares the various studies which have been done to study the prevalence and pattern of CAD in patients having bradyarrhythmias requiring PPI.
Various comorbidities and risk factors of CAD which were noted in our study population included hypertension (65%), diabetes-mellitus (28%), smoking (39%), angina (33%), dyslipidemia (35%) and family history of CAD (35%). Among different factors, dyslipidemia (56.7% vs 22%, p value =0.0016), family history of CAD (63.3% vs 18%, p value <0.0001) and angina (53.3% vs 20%, p value =0.0020) showed significant association with obstructive CAD. Similar pattern was observed in other studies from India [7][9].
In our study, RWMA was present in 50% of patients with CAD (27.5% of total population) while it was present in 14% of patients without CAD and the association was statistically significant (p value =0.0004). The mean left ventricular ejection fraction was lower in patients with associated obstructive CAD (52.7% vs 58.1%, p value =0.0270). Though echocardiography may be helpful and an effective non-invasive way to evaluate for the presence of CAD, there are other causes of RWMA on echocardiography including left bundle branch block and right ventricular pacing, which can decrease its specificity, necessitating CAG in patients with suspected CAD [14].
The causal association of CAD with conduction disorders is not well established and our data also does not prove any causality. But associated CAD can have important long term prognostic implications. In addition, it may be difficult to recognize ischemic changes on ECG after pacing especially with left bundle branch block pattern after RV apical pacing. Identifying and managing CAD earlier in patients who undergo PPI can have good impact on their long-term prognosis. The data also implies the need for larger multi-centre trials to understand the causal association and plan for earlier management.
Limitations. The notable limitations of this study include a smaller sample size and the study was carried out in a single centre. Extrapolating these results to general population needs validation from larger multi-centre studies. Effect of revascularization on bradyarrhythmias was not evaluated longitudinally. Long term follow-up studies are needed to quantify prognostic benefits in patients undergoing revascularization or receiving medical management for their associated CAD.
Conclusion
In patients requiring PPI, coexistent obstructive CAD was noted in more than one-third (37.5%) of the subjects. Causal association between obstructive CAD and conduction disturbances cannot be established from this data, but the increased prevalence of atherosclerotic cardiovascular disease in patients having bradyarrhythmias implies the need for larger multi-centre trials to understand the causal association and plan for earlier management. Need for basic science research identifying the possible link between conduction system disease and atherosclerotic cardiovascular disease is also established.
Relationships and Activities: none.
References
1. Patton KK, Olgin JE. Bradyarrhythmias and Atrioventricular Block. In: Braunwald's Heart Disease A Textbook of Cardiovascular Medicine. 12th ed. (International Edition): Libby P, Bonow RO, Mann DL, et al., 2022:1312-20. ISBN: 978-0-323-82468-2.
2. Kusumoto FM, Schoenfeld MH, Barrett C, et al. ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019; 140(8):e382-e482. doi:10.1161/CIR.0000000000000628. Erratum in: Circulation. 2019;140(8):e506-e508.
3. Knuuti J, Wijns W, Saraste A, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-77. doi:10.1093/eurheartj/ehz425. Erratum in: Eur Heart J. 2020;41(44):4242.
4. Das MK, Kumar S, Deb PK, et al. History of Cardiology in India. Indian Heart J. 2015; 67(2):163-9. doi:10.1016/j.ihj.2015.04.004.
5. Shenthar J, Bohra S, Jetley V, et al. A survey of cardiac implantable electronic device implantation in India: By Indian Society of Electrocardiology and Indian Heart Rhythm Society. Indian Heart J. 2016;68(1):68-71. doi:10.1016/j.ihj.2015.06.037.
6. Kalra A, Jose AP, Prabhakaran P, et al. The burgeoning cardiovascular disease epidemic in Indians — perspectives on contextual factors and potential solutions. Lancet Reg Health Southeast Asia. 2023;12:100156. doi:10.1016/j.lansea.2023.100156.
7. Alai MS, Beig JR, Kumar S, et al. Prevalence and characterization of coronary artery disease in patients with symptomatic bradyarrhythmias requiring pacemaker implantation. Indian Heart J. 2016;68 Suppl 3(Suppl 3):S21-S25. doi:10.1016/j.ihj.2016.06.013.
8. Mosseri M, Izak T, Rosenheck S, et al. Coronary angiographic characteristics of patients with permanent artificial pacemakers. Circulation. 1997;96(3):809-15. doi:10.1161/01.cir.96.3.809.
9. Vyas P, Meghnathi H, Joshi H, et al. Coexistent coronary artery disease in Indian patients undergoing permanent pacemaker implantation (PPI) for symptomatic bradyarrhythmia. Indian Heart J. 2021;73(5):577-81. doi:10.1016/j.ihj.2021.04.002.
10. Kumar D, Chakraborty R, Goutam S, et al. Incidence of Coronary Artery Disease After Permanent Pacemaker Implantation: A Hospital-based Study from East India. J Innov Card Rhythm Manag. 2024;15(6):5911-6. doi:10.19102/icrm.2024.15065.
11. Al-Hijji MA, Lennon RJ, Gulati R, et al. Safety and Risk of Major Complications With Diagnostic Cardiac Catheterization. Circ Cardiovasc Interv. 2019;12(7):e007791. doi:10.1161/CIRCINTERVENTIONS.119.007791.
12. India State-Level Disease Burden Initiative CVD Collaborators. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6(12):e1339-e1351. doi:10.1016/S2214-109X(18)30407-8.
13. Tandoğan I, Yetkin E, Güray Y, et al. Distribution of coronary artery lesions in patients with permanent pacemakers. Anadolu Kardiyol Derg. 2002;2(4):279-83.
14. Yavagal ST, Baliga VB. Non-Ischemic Regional Wall Motion Abnormality. Journal of The Indian Academy of Echocardiography & Cardiovascular Imaging. 2019;3(1):7-11. doi:10.4103/jiae.jiae_77_17.
15. Hsueh CW, Lee WL, Chen YT, et al. The incidence of coronary artery disease in patients with symptomatic bradyarrhythmias. Jpn Heart J. 2001;42(4):417-23. doi:10.1536/jhj.42.417.
16. Brueck M, Bandorski D, Kramer W. Incidence of coronary artery disease and necessity of revascularization in symptomatic patients requiring permanent pacemaker implantation. Med Klin (Munich). 2008;103(12):827-30. doi:10.1007/s00063-008-1130-z.
17. Yesil M, Arikan E, Postaci N, et al. Locations of coronary artery lesions in patients with severe conduction disturbance. Int Heart J. 2008;49(5):525-31. doi:10.1536/ihj.49.525.
18. Wei S, Zhong L, Chen S, et al. The status of coronary artery lesions in patients with conduction disturbance. J Cardiovasc Med (Hagerstown). 2011;12(10):709-13. doi:10.2459/JCM.0b013e328349187c.
19. Tayyebi M, Sani MD, Moghadam HRM, et al. Investigating the Manifestation of Coronary Artery Disease and Determining the Role of Effective Factors in the Need for Pacemaker Insertion in These Patients. Open Access Maced J Med Sci. 2019;7(13):2108-13. doi:10.3889/oamjms.2019.608.
About the Authors
A. B. WasilIndia
Senior Resident, Department of Cardiology, Nil Ratan Sircar Medical College and Hospital Kolkata
Competing Interests:
None
M. Kuila
India
Assistant Professor, Department of Cardiology, Nil Ratan Sircar Medical College and Hospital Kolkata
Competing Interests:
None
M. Mandal
India
Professor and Head, Department of Cardiology, Nil Ratan Sircar Medical College and Hospital Kolkata
Competing Interests:
None
D. khanra
United Kingdom
Queen Elizabeth Hospital, University Hospital Birmingham
Competing Interests:
None
R. K. Sharma
India
Professor and Ex HOD, Department of Cardiology, Nil Ratan Sircar Medical College and Hospital Kolkata
Competing Interests:
None
A. Mukherjee
United Kingdom
Specialty Registrar, Cardiology, York and Scarborough Teaching Hospitals NHS Foundation Trust
Competing Interests:
None
Supplementary files
Review
For citations:
Wasil A.B., Kuila M., Mandal M., khanra D., Sharma R.K., Mukherjee A. Does coronary artery disease go hand-in-hand with bradyarrhythmias? An observational study from Eastern India. Russian Journal of Cardiology. 2025;30(2):5949. https://doi.org/10.15829/1560-4071-2025-5949. EDN: PEYNTC

















































