Scroll to:
Effect of withdrawing long-term CPAP therapy on the course of obstructive sleep apnea
https://doi.org/10.15829/1560-4071-2021-4314
Abstract
Aim. To assess the effect of withdrawing long-term (12 months) CPAP therapy on the course of obstructive sleep apnea (OSA) in patients with moderate to severe disease.
Material and methods. The study included 40 patients with moderate to severe OSA and paroxysmal atrial fibrillation (AF) after surgical treatment. The mean age of the subjects was 59,3+8,2 years. In addition, 55% of patients had obesity. All patients were started on CPAP therapy. After 12 months, CPAP therapy was canceled in the patients who completed the study. Additional cardiorespiratory sleep monitoring was performed 1-2 days after the withdrawal of treatment to assess the severity of sleep-related breathing disorders.
Results. Cancellation of CPAP therapy in all observed cases led to an immediate relapse of OSA. Although the mean value of the apnea/hypopnea index (AHI) decreased from 24 episodes per hour [20; 34] before treatment up to 21 episodes per hour [13; 27] after 12-month CPAP therapy, there was no significant difference before and after therapy. In addition, the statistical analysis showed a transition from more severe OSA degrees to moderate ones, depending on the initial severity of the disease. Correlation analysis demonstrated significant relationships of the OSA severity, the final AHI value and the minimum oxygen level with the patient’s body weight (before and after therapy) (r=0,396, 0,411 and -0,488; r=0,358, 0,398 and -0,44, respectively, p<0,05).
Conclusion. In our study, when the 12-month CPAP therapy was canceled, no complete cure for sleep-related breathing disorders was recorded in any case. OSA recurrence was recorded immediately after discontinuation of CPAP therapy (on days 1-2) and its severity depended on the initial severity of the disease. At present, the continuation of CPAP therapy remains the only way to achieve complete control of OSA as a risk factor for AF.
Keywords
For citations:
Agaltsov M.V., Drapkina O.M. Effect of withdrawing long-term CPAP therapy on the course of obstructive sleep apnea. Russian Journal of Cardiology. 2021;26(2S):4314. https://doi.org/10.15829/1560-4071-2021-4314
Obstructive sleep apnea (OSA) is a very common disease characterized by transient upper airway collapse during sleep followed by hypoxemia, intrathoracic pressure swings and sleep fragmentation. The most widely cited study, the Wisconsin Sleep Cohort, showed an average of 10% prevalence of moderate to severe OSA (apnea-hypopnea index (AHI) >15 episodes per hour) among the population aged 30-70 years — 13% among men and 6% among women [1].
Creating a continuous positive airway pressure (CPAP) is currently the method of choice in many clinical situations in OSA treatment [2][3]. The action mechanism of this method is to create a continuous positive pressure and is aimed at overcoming the passive upper airway collapse [3]. This therapy is very effective in eliminating other pathological types of breathing during sleep (hypoventilation syndrome, upper airway resistance syndrome, snoring, in some
cases — central sleep apnea) and quickly normalizes their consequences [4]. Randomized trials in patients with moderate to severe OSA have shown that CPAP therapy reduces excessive daytime sleepiness, improves quality of life [5-9] and driver attentiveness [10]. In turn, cardiovascular morbidity and mortality, as has been shown in some studies, are reduced when using this therapy at least 4 hours per night [11].
The effectiveness of such treatment depends primarily on its correct use (at least 4 hours a night for 70% of nights). Even in patients with high adherence to therapy, the CPAP use can vary greatly [12][13], up to complete cessation of treatment. On the other hand, some patients with OSA stop the treatment on their own during the first year of therapy.
The data on disease course after discontinuation of CPAP therapy in literature is limited and contradictory. Some researchers believe that apnea does not immediately return to its original state during the first nights and even weeks after discontinuation of treatment [14-16]. These studies indicate that there may be residual effects of CPAP use that improve upper airway health. In addition, the transient desaturation characteristic of OSA may be less pronounced with therapy discontinuation than before initiation of therapy. Other studies have shown that abrupt withdrawal of CPAP therapy causes an immediate disease return with previous severity [17-20].
Thus, at present, questions remain open as to which extent OSA recurs after CPAP therapy cancellation, what factors will specify the severity of recurrence, and whether responders to CPAP therapy can be provided with other strategies for sleep-related breathing disorders.
Material and methods
Study cohort. This observational cohort 12-month study included patients with paroxysmal atrial fibrillation (AF) who underwent catheter treatment in the period from 2016 to 2018 at the National Medical Research Center for Therapy and Preventive Medicine. Starting from the 10th day after the operation, all patients underwent cardiorespiratory sleep monitoring (CRM) for one night to diagnose sleeprelated breathing disorders, and then patients with identified severe OSA were initiated automatic CPAP therapy for 12 months. The study cohort included 40 patients aged 37 to 75 years. After a year of continuous CPAP therapy, it was interrupted, and after 1-2 days, repeated CRM was performed to assess the severity of OSA recurrence.
The study was approved by the Ethics Committee of the National Medical Research Center for Therapy and Preventive Medicine. All patients signed written informed consent.
The study did not include patients with prior class ≥2 heart failure, decompensated underlying diseases, any cardiomyopathies, chronic obstructive pulmonary disease, neuromuscular diseases, as well as with current central sleep apnea >50% of all pathological respiratory events by CRM. In addition, voluntary withdrawal was considered an exclusion criterion.
Diagnosis of obstructive sleep apnea and selection of CPAP therapy. Sleep-related breathing disorders were diagnosed using a portable device for cardiorespiratory monitoring of sleep-related breathing disorders (Astrocard, Meditek, Russia). This device has 3rd diagnostic level, approved and recommended by the European Respiratory Society for the objective diagnosis of OSA [21]. The device allows registration of respiratory flow, respiratory movements of the chest or abdominal wall, electrocardiogram and blood oxygen level. After the study end, the data obtained were saved and then manually reviewed by a sleep medicine specialist using the Somno-Studio software (Meditek, Russia). Based on the current American Academy of Sleep Medicine (AASM) definitions and guidelines for OSA [22], obstructive apnea was defined as the airflow cessation 90% or more of the initial one with a duration ≥10 seconds while maintaining breathing efforts. Obstructive hypopnea was defined as a decrease (but not complete cessation) of air flow by 30% or more from the initial level lasting >10 seconds, which was accompanied by a drop in blood oxygen saturation by 3% or more while maintaining respiratory effort. Hypoxemia at night was defined as a decrease in mean blood oxygen saturation <92%. Nadir oxygen desaturation was defined as the minimum reduction in oxygen levels over the study night. The severity of OSA was determined by AHI presence, which reflects the number of apnea/ hypopnea episodes per study hour. Limits of normal range were AHI <5 ep/h. Mild OSA was defined with AHI of 5 to 14 ep/h, moderate OSA — AHI of 15 to 30 ep/h, and severe OSA — AHI >30 ep/h.
Non-invasive ventilation during sleep was carried out using a CPAP therapy devices (ResMed Autoset S9, Australia). Test CPAP therapy was initiated in automatic mode. The duration of test CPAP therapy ranged from 1 to 4 days. At the end of test CPAP therapy, the data were transferred to ResScan software (ResMed, Australia). The therapy was considered effective, as a result of which either a complete OSA remission was achieved (residual AHI <5 ep/h), or a decrease in apnea episode number by 50% or more from the initial value. Further, manual correction of device settings for long-term CPAP therapy was carried out. Patients received appropriate recommendations for using the device (using at least 5 days per week with duration at least 4 hours during the night), humidifier, nasal mask, and other components. The CPAP treatment parameters were monitored 3, 6 and 12 months after starting therapy.
Within 12 months, 7 patients left the study (2 — during recruitment, 3 — at visit after 3 months, and 2 — at visit after 6 months). There were following reasons for leaving the study: subjective intolerance to treatment — 2 patients, voluntary refusal to participate in the study — 4 patients, inability to use treatment for personal reasons — 1 person).
After 12 months, in 33 patients who completed the study, CPAP therapy was discontinued. One-two days after, repeated CRM was performed, the results of which were processed similarly to the protocol of first examination.
Statistical analysis. Quantitative variables were described by the following parameters: number of patients, arithmetic mean (M), standard deviation (δ), 25th and 75th percentiles, and median. Qualitative variables were described by absolute and relative frequencies. Differences were considered at p<0,05.
For quantitative variables, the Kolmogorov-Smirnov test for normality was carried out. The results were evaluated using parametric and nonparametric statistics: Pearson χ2 test, two-way Friedman test, unpaired Student t-test, nonparametric Mann-Whitney, Wilcoxon, Kruskal-Wallis tests. To determine the mutual influence of indicators, Spearman’s correlation was used.
The calculation was performed using the Microsoft Excel and the Statistica 10 for Windows (StatSoft Inc., USA).
Results
Table 1 summarizes the baseline characteristics prior to treatment. So, 55% of patients had obesity of various classes. There was almost similar sex distribution (men, 52,5%). The mean age was 59,3±8,2 years, while persons over 60 years old prevailed (52,8%) (at this age, the disease is detected in men and women with the same frequency). In addition, there was a relatively small number of smokers (22,5%). The overwhelming majority of patients had hypertension and received antihypertensive therapy (92,3% and 82,5%, respectively). Also, 62,5% of those included in the study had an enlarged left atrium (>40 mm), which is a risk factor for AF recurrence).
Table 1
Characteristics of patients receiving CPAP therapy
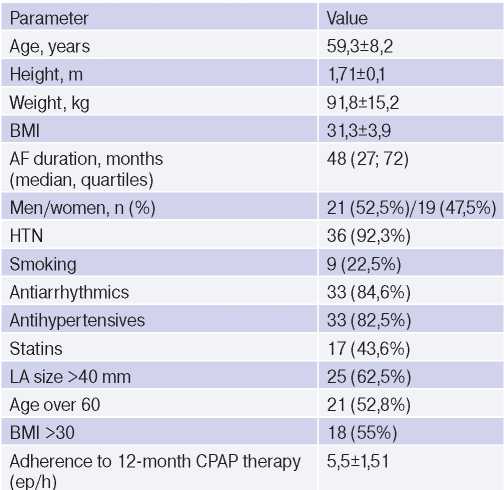
Abbreviations: HTN — hypertension, BMI — body mass index, LA — left atrium, AF — atrial fibrillation.
As Figure 1 shows, test CPAP therapy transferred moderate and severe OSA to complete remission (two-way Friedman test, p=0,001). In one case, the number of apnea decreased by 50% from the baseline, which is also a criterion for successful CPAP therapy. Disease remission persisted during all further visits. However, the cancellation of CPAP therapy in all cases led to an immediate relapse of OSA — the median AHI was 21 [ 13; 27 ] ep/h. Although the AHI decreased compared to the baseline values (24 [ 20; 34 ]), no significant difference was obtained.
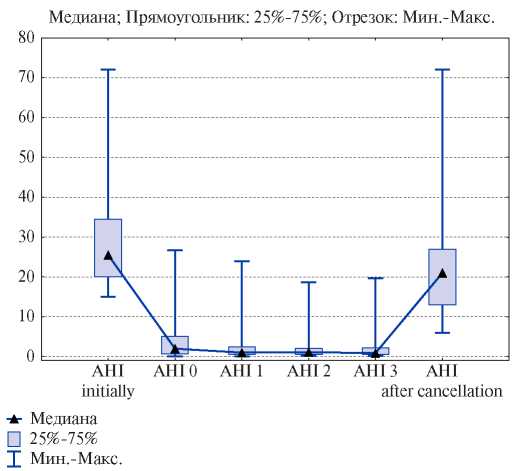
Figure 1. Dynamics of AHI before, during and after the cancellation of CPAP therapy.
Abbreviations: AHI — Apnea-Hypopnea Index.
Also, no significant improvement (Figure 2) was noted in average and minimum value (nadir) of oxygen at night after CPAP withdrawal.
We performed a paired analysis comparing the initial severity of OSA before treatment and after discontinuation of CPAP therapy (Figure 3). In the group with initially moderate OSA, in 10 patients (52,6%) after CPAP discontinuation, the severity level decreased to mild, while in 8 (42,1%) patients it did not change, and in one patient it became severe. In the group with initially severe OSA, the transition to a mild OSA did not occur in any patient (severity either did not change (28,6%), or moved to moderate severity category (71,4%).
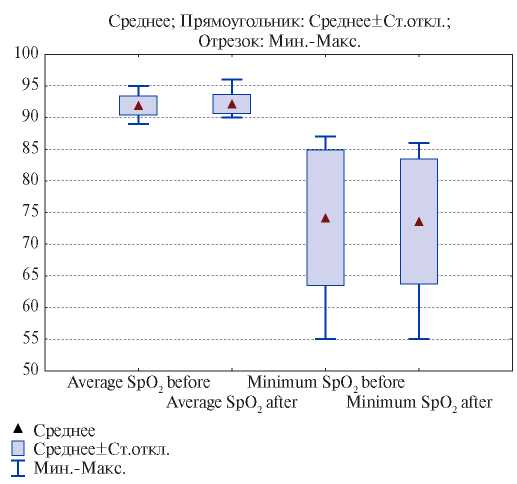
Figure 2. Dynamics of the average and minimum blood oxygen level (nadir) before and after CPAP therapy.
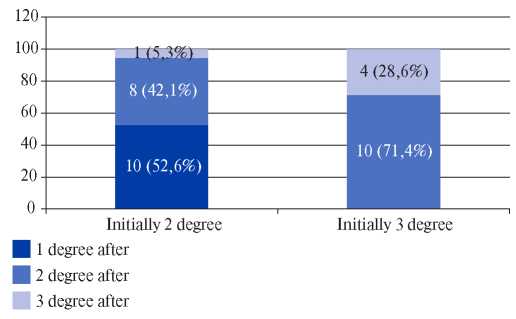
Figure 3. Severity of OSA after treatment cancelation versus baseline.
Correlation analysis of OSA severity, levels of saturation, height, weight, body mass index, hypertension presence showed that there is a significant dependence of disease severity, final AHI and nadir oxygen desaturation on body weight (before and after therapy) (r=0,396, 0,411 and -0,488; r=0,358, 0,398 and -0,44, respectively, p<0,05). It should be noted that we did not obtain a significant correlation between compliance to therapy and OSA recurrence after therapy discontinuation.
Discussion
In modern guidelines, the appointment of CPAP therapy are described in sufficient detail, however, its duration, the possibility of complete cancellation or replacement with another type of therapy are not specified [3].
Discontinuation of CPAP therapy has been studied in several studies where its duration ranged from one night to 2 weeks. Some researchers report a OSA recurrence after discontinuation of CPAP therapy, but with a lesser degree of severity than before starting treatment. For example, Kribbs, et al. found that CPAP withdrawal for one night resulted in AHI averaging 34 ep/h compared to 57 ep/h before treatment [23]. Other authors have shown a return to baseline AHI values on the first night of CPAP therapy withdrawal. However, it was accompanied by a smaller drop in blood oxygen levels during sleep after the first night of withdrawal. Further, 1 week after therapy cancellation, oxygen levels returned to baseline severity [16]. A randomized study comparing CPAP withdrawal for a longer period (2 weeks with subsequent continuation of therapy) showed a rapid OSA recurrence, characterized by a return to baseline AHI within the first night after discontinuation, but with a less pronounced drop in the blood oxygen level in the first night of cancellation [15]. Recent study noted that OSA did not recur at all in 1/3 of patients after 4 nights of CPAP withdrawal, but this regarded patients with initially less severe OSA and less body weight [14]. This study ranks among those confirming the absence of OSA recurrence during acute CPAP withdrawal.
In our study, we did not receive such a pronounced response after 12-month CPAP therapy. However, the mean AHI decreased compared to the baseline, this decrease was not significant. Also, no significant improvement in blood gas levels has been shown. Neither the average oxygen value per night nor the nadir desaturation changed after CPAP therapy immediately after its cancellation. In general, our data did not differ from the data of most previous studies, since without treatment, the apnea severity and blood gas composition invariably returned to baseline after a fairly short period of time (from 1-2 days to 2 weeks).
What factors could theoretically reduce OSA recurrence after discontinuation of treatment? There are studies showing that the decrease in OSA severity may simply reflect the known variability between different nights, which, in turn, determines recurrence in the lower ranges of disease severity [24-26]. However, this variability becomes less likely or completely absent in severe OSA. Nevertheless, with a very severe initial OSA, long-term CPAP therapy can contribute to regression to the mean AHI values. This fact was reflected in our study, when the majority of patients with severe OSA (71,4%) moved to the group of moderate severity.
It could be expected that in patients with increasing age and obesity, remission of OSA after discontinuation of CPAP therapy would be unlikely. This fact is confirmed by our data (in 55% of patients, body mass index and mean age were >30 kg/m2 and 59,3±8,2 years, respectively).
In addition, sleep apnea severity can be influenced by such factors as upper airway edema under the influence of chronic barotrauma — periodic snoring during apnea [27], nocturnal rostral fluid shift [28], impaired respiratory sensory afferent reflexes [29] and hyoid muscle weakness [30]. A reproducible effect of CPAP therapy on apnea severity can be expected if it results in a reduction in the effects of these factors. In some studies, after long-term CPAP therapy, its effect was assessed using magnetic resonance imaging [31]. The anatomical remodeling effect of non-invasive ventilation during sleep on upper respiratory tract structures has been demonstrated. It has also been shown in a number of studies that CPAP therapy can reduce the effect of respiratory center during apnea and reduce respiratory responses that are responsible for upper airway respiratory instability [32][33].
The last factor affecting the possibility of remission after the CPAP withdrawal is the duration and regularity of its use. In our study, 12-month use of CPAP therapy did not show a significant effect on remission after discontinuation of treatment.
There are certain limitations in our study. First, we did not conduct a polysomnography with a record of sleep patterns. It is known that the cancellation of CPAP therapy negatively affects the sleep architecture, leads to an increase in stages of wakefulness and superficial slow-wave sleep and to a decrease in time of paradoxical sleep (REM sleep). Sleep instability with frequent awakenings and EEG activation reactions usually aggravates OSA course [34]. Second, we did not assess daytime functions (primarily daytime sleepiness) after CPAP therapy was discontinued. The data on the effects of CPAP withdrawal on objective and subjective daytime sleepiness are contradictory. In studies [12], it was shown that when CPAP therapy was canceled, sleepiness immediately returned to its pre-treatment levels. This was the same for both severe and moderate OSA. In contrast, Sforza et al. [35] found that subjective sleepiness did not return to baseline levels. Although it is possible that the differences can be explained by the influence of weight loss on final result.
There is also evidence that CPAP withdrawal has shown a relationship between OSA recurrence and impaired endothelial function, an increase in urine concentration of catecholamines, an increase in blood pressure and heart rate, i.e. resumption of increased sympathetic activity characteristic of untreated OSA [15][36].
The issue of replacing or completely discontinuing CPAP therapy with a decrease in the severity of OSA after treatment is extremely poorly covered in the literature. On the one hand, our patients require constant supervision of breathing-related sleep disorders, because OSA is combined with a paroxysmal AF, which required surgical intervention. Modern studies indicate a high incidence of obstructive breathing disorders during sleep in patients with various AF types [37][38]. Current guidelines and consensus papers consider OSA as a risk factor for development and/or recurrence of AF and suggest continuous long-term CPAP in order to reduce the postoperative risk of AF [39][40]. In clinical guidelines for other types of OSA treatment [41], it is possible to replace CPAP therapy with intraoral devices in cases of moderate and severe OSA if it is impossible to continue CPAP treatment for various reasons.
Thus, there is no convincing evidence on complete cure for respiratory disorders when OSA treatment is interrupted. OSA recurs to one degree or another in the near future after CPAP therapy is discontinued. Therefore, continuation of CPAP therapy currently remains the only way to achieve complete and controlled OSA remission. Replacing CPAP therapy with another method of treating OSA is theoretically possible, but requires additional research to prove the effectiveness of such a replacement.
Conclusion
In our study, when the 12-month CPAP therapy was canceled, no complete cure for sleep-related breathing disorders was recorded in any case. OSA recurrence was recorded immediately after discontinuation of CPAP therapy (on days 1-2) and its severity depended on the initial severity of the disease. At present, the continuation of CPAP therapy remains the only way to achieve complete control of OSA as a risk factor for AF.
References
1. Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006-14. doi:10.1093/aje/kws342.
2. Khayat R, Pleister A. Consequences of Obstructive Sleep Apnea: Cardiovascular Risk of Obstructive Sleep Apnea and Whether Continuous Positive Airway Pressure Reduces that Risk. Sleep Med Clin. 2016;11:273-86. doi:10.1016/j.jsmc.2016.05.002.
3. Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335-43. doi:10.5664/jcsm.7640.
4. Salord N, Mayos M, Miralda RM, et al. Continuous positive airway pressure in clinically stable patients with mild-to-moderate obesity hypoventilation syndrome and obstructive sleep apnoea. Respirology. 2013;18(7):1135-42. doi:10.1111/resp.12131.
5. Osman AM, Carberry JC, Burke PGR, et al. Upper airway collapsibility measured using a simple wakefulness test closely relates to the pharyngeal critical closing pressure during sleep in obstructive sleep apnea. Sleep. 2019;42(7):zsz080. doi:10,1093/sleep/zsz080.
6. Schwarz Е!, Stradling J R, Kohler M. Physiological consequences of CPAP therapy withdrawal in patients with obstructive sleep apnoea — an opportunity for an efficient experimental model. J Thorac Dis. 2018;10(1):S24-S32. doi:10.21037/jtd.2017.12.142.
7. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61(5):430-4. doi:10.1136/thx.2005.050583.
8. Inoue Y, Miki M, Tabata T. Findings of the Maintenance of Wakefulness Test and its relationship with response to modafinil therapy for residual excessive daytime sleepiness in obstructive sleep apnea patients adequately treated with nasal continuous positive airway pressure. Sleep Med. 2016;27-28:45-8. doi: 10.1016/j.sleep.2016.06.035.
9. Elfimova EM, Mikhailova OO, Khachatrian NT, et al. The effect of adherence with longterm PAP therapy on the psycho-emotional state of patients with obstructive sleep apnea syndrome. Systemic Hypertension. 2020;17(2):56-60. (In Russ.) doi: 10.26442/2075082X.2020.2.200176.
10. McCall CA, Watson NF. Sleepiness and Driving: Benefits of Treatment. Sleep Med Clin. 2019;14(4):469-78. doi:10.1016/j.jsmc.2019.07.001.
11. Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115-22. doi:10.7326/0003-4819-156-2-201201170-00006.
12. Young LR, Taxin ZH, Norman RG, et al. Response to CPAP withdrawal in patients with mild versus severe obstructive sleep apnea/hypopnea syndrome. Sleep. 2013;36(3):405-12. doi:10.5665/sleep.2460.
13. Jun JC, Unnikrishnan D, Schneider H, et al. Effect of Acute Intermittent CPAP Depressurization during Sleep in Obese Patients. PLoS ONE. 2016;11(1):e0146606. doi:10.1371/journal.pone.0146606.
14. Rossi VA, Schwarz EI, Bloch KE, et al. Is continuous positive airway pressure necessarily an everyday therapy in patients with obstructive sleep apnoea? Eur Respir J. 2014;43:1387-93. doi:10.1183/09031936.00180213.
15. Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192-9. doi:10.1164/rccm.201106-0964OC.
16. Yang Q, Phillips CL, Melehan KL, et al. Effects of short-term CPAP withdrawal on neurobehavioral performance in patients with obstructive sleep apnea. Sleep. 2006;29:545-52. doi:10.1093/sleep/29.4.545.
17. Hevener B, Hevener W. Continuous Positive Airway Pressure Therapy for Obstructive Sleep Apnea. Sleep Medicine Clinics. 2016;11(3):323-9. doi:10.1016/j.jsmc.2016.04.004.
18. Fiz JA, Abad J, Ruiz J, et al. nCPAP treatment interruption in OSA patients. Respir Med. 1998;92:28-31. doi:10.1016/s0954-6111(98)90028-2.
19. Vroegop AV, Smithuis JW, Benoist LB, et al. CPAP washout prior to reevaluation polysomnography: a sleep surgeon's perspective. Sleep Breath. 2015; 19(2):433-9. doi:10.1007/s11325-014-1086-6.
20. Boudewyns A, Sforza E, Zamagni M, et al. Respiratory effort during sleep apneas after interruption of long-term CPAP treatment in patients with obstructive sleep apnea. Chest. 1996;110:120-7. doi:10.1378/chest.110.1.120.
21. Parati G, Lombardi C, Hedner J, et al. EU COST Action B26 members Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J. 2013;41:523-38. doi:10.1183/09031936.00226711.
22. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506.
23. Kribbs NB, Pack AI, Kline LR, et al. Effects of one night withoutnasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162-8. doi:10.1164/ajrccm/147.5.1162.
24. Le Bon O, Hoffmann G, Tecco J, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118:353-9. doi:10.1378/chest.118.2.353.
25. Skiba V, Goldstein C, Schotland H. Night-to-Night Variability in Sleep Disordered Breathing and the Utility of Esophageal Pressure Monitoring in Suspected Obstructive Sleep Apnea. J Clin Sleep Med. 2015;11(6):597-602. doi:10.5664/jcsm.4764.
26. Ahmadi N, Shapiro GK, Chung SA, et al. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13:221-6. doi:10.1007/s11325-008-0234-2.
27. Bassiouny A, Nasr S, Mashaly M, et al. Electron microscopy study of peripheral nerves in the uvulae of snorers and obstructive sleep apnoea patients. The Journal of Laryngology and Otology. 2009;123(2):203-7. doi:10.1017/S0022215108002971.
28. White LH, Lyons OD, Yadollahi A, et al. Night-to-night Variability in Obstructive Sleep Apnea Severity: Relationship to Overnight Rostral Fluid Shift. J Clin Sleep Med. 2015;11:149-56. doi:10.5664/jcsm.4462.
29. Daulatzai MA. Role of sensory stimulation in amelioration of obstructive sleep apnea. Sleep Disord. 2011:596879. doi:10.1155/2011/596879.
30. McSharry D, O'Connor C, McNicholas T, et al. Genioglossus fatigue in obstructive sleep apnea. Respir Physiol Neurobiol. 2012;183:59-66. doi:10.1016/j.resp.2012.05.024.
31. Shrivastava D. Impact of sleep-disordered breathing treatment on upper airway anatomy and physiology. Sleep Med. 2014;15(7):733-41. doi:10.1016/j.sleep.2014.01.002.
32. Strohl KP, Wellman A. When CPAP is stopped: what are the “on switches” of sleep apnoea? Eur Respir J. 2014;43:1227-9. doi:10.1183/09031936.00003614.
33. Loewen A, Ostrowski M, Laprairie J, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32:1355-65. doi:10.1093/sleep/32.10.1355.
34. Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996-1004. doi:10.1164/rccm.201303-0448OC.
35. Sforza E, Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep. 1995;18:195-201.
36. Thiel S, Haile SR, Peitzsch M, et al. Endocrine responses during CPAP withdrawal in obstructive sleep apnoea: data from two randomised controlled trials. Thorax. 2019;74(11):1102-5. doi:10.1136/thoraxjnl-2019-213522.
37. Youssef I, Kamran H, Yacoub M, et al. Obstructive Sleep Apnea as a Risk Factor for Atrial Fibrillation: A Meta-Analysis. J Sleep Disord Ther. 2018;7:282. doi:10.4172/2167-0277.1000282.
38. Agaltsov MV, Drapkina OM, Davtyan KV, et al. The prevalence of sleep breathing disorders in patients with atrial fibrillation undergoing catheter treatment. Rational Pharmacotherapy in Cardiology. 2019;15(1):36-42. (In Russ.) doi: 10.20996/1819-6446-2019-15-1-36-42.
39. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498. doi:10.1093/eurheartj/ehaa612.
40. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):е275-е444. doi:10.1016/j.hrthm.2017.05.012.
41. Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773-827. doi:10.5664/jcsm.4858.
About the Authors
M. V. AgaltsovRussian Federation
Moscow
Competing Interests:
not
O. M. Drapkina
Moscow
Competing Interests:
not
Supplementary files
Review
For citations:
Agaltsov M.V., Drapkina O.M. Effect of withdrawing long-term CPAP therapy on the course of obstructive sleep apnea. Russian Journal of Cardiology. 2021;26(2S):4314. https://doi.org/10.15829/1560-4071-2021-4314

















































