Перейти к:
Избыточная масса тела и ожирение при метаболическом синдроме
https://doi.org/10.15829/1560-4071-2025-6535
Аннотация
В настоящей статье проанализированы современные представления о патогенезе, диагностике и влиянии на прогноз избыточной массы тела при метаболическом синдроме. Приведен обзор современных возможностей диагностики, немедикаментозной и медикаментозной терапии избыточной массы тела, а также сердечно-сосудистых преимуществ препаратов, используемых для снижения массы тела.
Ключевые слова
Для цитирования:
Недогода С.В., Цыганкова О.В. Избыточная масса тела и ожирение при метаболическом синдроме. Российский кардиологический журнал. 2025;30(1S):6535. https://doi.org/10.15829/1560-4071-2025-6535
For citation:
Nedogoda S.V., Tsygankova O.V. Overweight and obesity in metabolic syndrome. Russian Journal of Cardiology. 2025;30(1S):6535. (In Russ.) https://doi.org/10.15829/1560-4071-2025-6535
Диагностика и лечение избыточной массы тела (ИзМТ) и ожирения у лиц с метаболическим синдромом (МС) проводится в соответствии с действующими клиническими рекомендациями1. Вместе с тем представляется целесообразным более подробно остановиться на ключевых особенностях ведения этого фенотипа пациентов.
Эпидемиология
Если ожирение рассматривать как основной и обязательный критерий МС, то естественно, что его частота встречаемости будет составлять 100%. Однако в последние годы стали выделять фенотип пациентов с МС без ИзМТ [1-3] и он составляет от 10 до 20% [4-6].
Особенности патогенеза
В самом упрощенном варианте развитие ожирения является результатом дисбаланса между поступлением энергии и энерготратами, но в реальности это очень сложный по патогенезу процесс [7], включающий в себя взаимодействие между генетическими и психологическими факторами и факторами окружающей среды [8]. Энергетический баланс обеспечивается комплексом сложных взаимосвязей между центральной нервной системой, жировой и мышечной тканью, кишечника, печени и поджелудочной железы.
Генетика
Вклад генетических факторов в развитие ожирения может составлять 40-70% [9][10] и связан с более 300 локусами хромосом [8], но при этом они обуславливают всего ~5% вариаций индекса массы тела (ИМТ) [11, 12].
Краткосрочная регуляция энергетического баланса
Гипоталамус играет важную роль в регуляции метаболизма [13], интегрируя сигналы о энергетических запасах и поступлении энергии через контроль потребления пищи, физической активности и основных базовых энергозатратах [14]. Краткосрочное пищевое поведение также контролируется структурами заднего мозга, где tractus solitarius получает информацию через афферентные пути nervus vagus, стимулируемые секретином и холецистокинином [15].
Аппетит стимулирующие нейроны в дугообразном ядре содержат нейропептид Y, стимулирующий Y рецепторы (Y1 и Y5), агути-связанный пептид, являющийся антагонистом активности MC3/4 рецепторов. Эти белки вместе с эндоканабиоидами являются стимуляторами аппетита. Грелин, преимущественно вырабатывающийся в желудке, также повышает аппетит.
Нейропептид Y играет важнейшую роль в поддержании энергетического гомеостаза [16] и является одним из наиболее мощных стимуляторов аппетита [17]. Нейропептид Y также продуцируется нейронами симпатической нервной системы и вызывает вазоконстрикцию, а также способствуют увеличению массы жировой ткани. Отрицательный энергетический баланс повышает уровень нейропептида Y в гипоталамусе, что увеличивает потребление пищи при уменьшении энерготрат за счет подавления симпатической активности. Кроме этого, изменение уровня нейропептида Y приводит к изменению настроения и тревожности [16][18].
Агути-связанный пептид. Агути-связанный пептид является стимулирующим аппетит нейропептидом, который влияет на те же нейроны, что и нейропептид Y. Они активируются при отрицательном энергетическом балансе (например, голодании) и при снижении уровня лептина и инсулина, оказывающих ингибирующее влияние на эти нейроны [19]. Активация агути-связанных нейронов приводит к повышенному потреблению пищи для восстановления дефицита поступления энергии [20] и при этом может формироваться механизм "вознаграждения" при потреблении пищи на фоне голодания [21][22].
Грелин. Самый высокий уровень грелина отмечается при голодании и непосредственно перед приемом пищи [23]. Грелин вырабатывается клетками желудочно-кишечного тракта (ЖКТ), их наибольшая плотность наблюдается в дне желудка [24][25]. В гипоталамусе грелин активирует те же нейроны, что и нейропептид Y и агути-связанный пептид [26] и стимулирует аппетит. Грелин также стимулирует выработку гормона роста [27]. Изменения рецепторов грелина могут быть связаны с генетическими мутациями [28-31]. Вместе с тем при ожирении часто отмечается снижение уровня грелина и его повышение при снижении веса [32]. При анорексии на фоне онкологической патологии введение грелина увеличивает потребление пищи [33]. Вероятно, стимуляция выработки грелина определяется не только ожирением, но и эндокринным статусом в целом.
Эндоканнабиноиды. Открытие эндоканнабиодной системы продемонстрировало ее важную роль в регуляции аппетита, пищевого поведения и регуляции энергетического баланса и массы тела [34]. CB1-рецепторы локализованы в мозге (преимущественно в гипоталамусе и лимбической системе) и регулируют поступление пищи [35]. Они также активируют метаболизм жиров в белой жировой ткани и глюкозы. CB2-рецепторы преимущественно представлены в иммунных клетках и играют важную роль в формировании иммунного ответа [36].
Секретин. Активация секретина через периферические (n. vagus) и центральные механизмы приводит у анорексигенному эффекту [37], активации термогенеза и липолизу бурой жировой ткани [37][38].
Холецистокинин. Холецистокинин ("гормон сытости") вырабатывается в подвздошной и двенадцатиперстной кишке [39]. Стимулом его образования является наличие пищи в желудке, и он способствует прекращению приема пищи [40] через активацию афферентных нейронов и усиление активности nucleus tractus solitary [41]. Часть из этих нейронов заднего мозга влияет через парабрахиальное ядро (ключевое звено регуляции аппетита) на секрецию глюкагоноподобного пептида-1 [42].
Долгосрочная регуляция энергетического баланса
Ключевыми факторами долгосрочной регуляции энергетического баланса являются лептин и инсулин.
Лептин. Лептин — это адипокин, секретируемый белой жировой тканью. Его концентрация прямо пропорциональна количеству жировой ткани. Лептин регулирует чувство насыщения и энерготраты через стимуляцию проопиомеланокортина и подавление нейропептида Y/агути-связанного пептида в гипоталамусе. Нарушение продукции лептина может быть обусловлено мутациями его гена или изменениями родственных ему рецепторов, приводящими к гиперфагии и развитию лептинорезистентности. Возможно, что лептин в большей степени препятствует снижению жировых отложений, чем способствует их накоплению.
Инсулин. Уровень инсулина прямо коррелирует с массой жировой ткани. Его высокий уровень через механизм обратной связи с центральной нервной системой способствует снижению потребления пищи. При ожирении имеет место инсулинорезистентность и гиперинсулинемия, связанные с повышенным уровнем свободных жирных кислот и подавлением липолиза [43].
Инкретины. Инкретины (глюкозозависимый инсулинотропный полипептид (ГИП) и глюкагоноподобный пептид-1 (ГПП-1)) — это пептиды, секретируемые энтеро-эндокринными клетками в ЖКТ в ответ на прием пищи, стимулирующий секрецию инсулина. Они обеспечивают феномен инкретинового эффекта, когда при пероральном приеме глюкозы секреция инсулина в 2-3 раза больше, чем при ее внутривенном введении [44]. ГИП стимулирует секрецию глюкагона, особенно при низком уровне глюкозы, в то время как ГПП-1 подавляет секрецию глюкагона, особенно при гипергликемии [44][45]. ГИП повышает инсулин стимулированный транспорт глюкозы, синтез жирных кислот и их включение в триглицериды [17]. ГПП-1 влияет на функцию многих органом и систем, снижает аппетит и потребление пищи, что приводит к снижению веса. ГИП и ГПП-1 быстро разрушаются ферментом дипептидилпептидазой 4 [46].
Амилин. Амилин является полипептидным гормоном, секретируемым параллельно с инсулином β-клетками поджелудочной железы [47]. Он также продуцируется в латеральном гипоталамусе и в синергии с леп- тином уменьшает потребление энергии [48]. Кроме того, амилин увеличивает энерготраты и влияет на пристрастия к определенной пищи ("пищевой гедонизм") [49][50].
Роль белой жировой ткани
Белый жир — основа жировой ткани. Он бывает подкожным и висцеральным. Подкожный жир копится в так называемых "ловушках": на животе, боках, ягодицах. Висцеральный окружает сердце и органы брюшной полости и при избытке сдавливает их, оказывая негативное влияние на их функции. Белая жировая ткань метаболически активна, продуцируя более 50 адипокинов (первыми были открыты адипонектин, лептин и резистин), влияющих на метаболический гомеостаз, липидный обмен, воспаление и иммунную функцию [51].
Снижение уровня адипонектина играет важную роль при ожирении в сочетании с коморбидной патологией [52]. Он обладает антиатеросклеротическим эффектом, усиливает окисление жирных кислот, подавляет продукцию глюкозы печенью [53]. Адипонектин способствует снижению веса, увеличивая энерготраты [54]. Противоположные адипонектину эффекты оказывает резистин, вероятнее всего за счет усиления инсулинорезистентности [55-57]. Роль других адипокинов [58] менее изучена, хотя уже продемонстрировано влияние хемерина, липокалина-2, васпина и оментина-1 на иммунитет и воспаление [58].
Роль бурой жировой ткани
Бурая жировая ткань играет важную роль в процессах термогенеза, стимулирует энерготраты, уменьшает инсулинорезистентность и способствует снижению веса [59-61]. Среди факторов, способствующих образованию бурой жировой ткани, помимо низкой температуры, необходимо выделить стимуляцию β3-адренорецепторов. Адипокины, продуцируемые бурой жировой тканью, оказывают кардиопротективное действие [62] и активируют липолиз [63].
Особенности течения
При анализе длительности существования ожирения (20 лет (очень длительное ожирение), 8 лет (длительное ожирение), 6 лет (недавнее ожирение) и 3 года (вновь возникшее ожирение)) наихудший метаболический профиль (инсулинорезистентность, повышение высокочувствительного С-реактивного белка (СРБ) и снижение липопротеинов высокой плотности) по сравнению с лицами без ожирения был при недавно возникшем ожирении и длительном ожирении [64].
Особенности диагностики
Антропометрия
ИМТ
Повышенным считается1 ИМТ ≥25 кг/м2.
Расчет показателя ИМТ (индекс Кетле, рассчитываемый по формуле: вес (кг): рост (м) = (кг/м2)), был предложен бельгийским математиком Адольфом Кетле в 1869г. Несомненными достоинствами этого показателя являются его абсолютная доступность, безопасность и практически нулевая стоимость [65] и использование при базовом обследовании практически всех пациентов. Однако опыт его применения при скрининге пациентов с ИзМТ и ожирением выявил снижение его диагностической и прогностической ценности у некоторых фенотипов пациентов (спортсмены с большой мышечной массой, при старческой астении) и этносов [66][67]. Но при этом показатель ИМТ остается самым часто используемым показателем при скрининге, диагностике и оценке метаболических и сердечно-сосудистых рисков (ССР) [68]. Комбинация показателя ИМТ с окружностью талии (ОТ), объёма бедер (ОБ), отношением ОТ/ОБ, процентом жировых отложений повышает его диагностическую и прогностическую ценность.
ОТ
Наличие абдоминального ожирения диагностируется при ОТ ≥80 см у женщин и ≥94 см у мужчин европейской расы.
ОТ определяется при измерении сантиметровой лентой (она плотно прилегает, но не сдавливает и человек не задерживает дыхание и не втягивает живот во время исследования) вокруг талии в самой узкой ее части. При этом, согласно различным рекомендациям, имеются различия в топографии определения ОТ: согласно Всемирной организации здравоохранения (ВОЗ), измерение рекомендуется проводить по средней точке между нижним краем последнего прощупываемого ребра и верхней частью гребня подвздошной кости [69], а по Inter ASIA International Collaboration on Cardiovascular Disease на 1 см выше пупка [70]. Измерение проводится на голое тело или через тонкую одежду при отсутствии метеоризма и спустя не менее часа после приема пищи.
Этот показатель позволяет легко диагностировать абдоминальное (центральное) ожирение, которое прямо коррелирует с выраженностью висцерального ожирения, повышением ССР и онкологического риска, МС, сахарного диабета (СД) 2 типа и коморбидной патологии [71-73].Определение ОТ рекомендовано для скрининга ожирения1, этот показатель более точен при диагностике абдоминального ожирения, чем показатели ИМТ и ОТ/ОБ [74]. На точность определения ОТ влияют рост (высокий и низкий) человека, выраженные мышцы брюшного пресса и беременность [75].
ОБ
Для мужчин с ростом 170-176 см ОБ в пределах 89-96 см считается нормальным, а для мужчин ростом от 170 до 192 см нормальный ОБ от 92 до 118 см. Увеличение ОБ >101,5 см у женщин при ИМТ >28 кг/м2 расценивается как наличие у них ожирения [38] и является фактором риска развития метаболических осложнений [76], сердечно-сосудистых заболеваний (ССЗ), ишемической болезни сердца (ИБС), СД 2 типа и преждевременной смерти [77][78]. В сочетании с ИМТ может быть методом скрининга ожирения. Для измерения ОБ необходимо обернуть сантиметровую ленту вокруг бедер по самой широкой части, учитывая выступающие точки ягодиц и живота. При наличии выступающего живота, при наложении ленты необходимо ее учитывать. Информативность показателя снижается у лиц с выраженными мышцами бедра.
Индекс ожирения тела (ИОТ)
ИОТ был предложен Bergman RN, et al. [79] и рассчитывается по формуле: ОТ (см)/рост (м)1,5-18 и чаще используется у латино- и афроамериканцев [80]. Считается, что ИОТ прямо коррелирует с уровнем мочевой кислоты, триглицеридов, глюкозы натощак и с другими антропометрическими показателями (ИМТ, ОТ, ОТ/ОБ) [81]. Его прогностическая ценность при ожирении ниже, чем у ИМТ и ОТ, но выше, чем у ОТ/ОБ [82]. Информативность показателя низкая при объеме жировых отложений <15% или при экстремальном ожирении [83].
ОТ/ОБ
Был предложен ВОЗ для определения абдоминального ожирения и более точного прогнозирования ССР [84], но из-за различных методик измерения ОТ недостаточно стандартизирован. По нормам ВОЗ ОТ/ОБ ≥0,90 у мужчин и ≥0,85 у женщин свидетельствует о наличии абдоминального ожирения [85]. Более "громоздкий" показатель ОТ/ОБ имеет те же недостатки, что и его отдельные составляющие [86], но он более информативен, чем ИМТ [87]. Считается, что ОТ/ОБ прямо коррелирует с уровнем мочевой кислоты, глюкозы натощак, уровнем артериального давления (АД) и нарушениями липидного обмена [87]. Имеющиеся данные свидетельствуют о высокой прогностической значимости этого показателя в отношении развития инфаркта миокарда [88] и обструктивного сонного апноэ (ОСА) [89].
Объем шеи (ОШ)
ОШ измеряется по основанию сантиметровой лентой таким образом, чтобы она проходила ниже Адамова яблока над яремной впадиной спереди и по основанию шеи сзади. У мужчин нормальный объем шеи ~40,5 см, а у женщин — ~34,2 см, но вариабельность данного показателя сильно зависит от типа телосложения. ОШ отражает выраженность подкожных и висцеральных жировых отложений верхней половины тела [90]. ОШ — хороший предиктор развития МС, СД 2 типа, сердечно-сосудистых осложнений, ОСА и гиповентиляционного синдрома [91, 92]. Показатель имеет существенные межрасовые различия.
Толщина кожной складки
Может определяться в разных местах: по методике Коровина толщина кожной складки измеряется на уровне III ребра (в норме 1-1,5 см) и параумбиликально, сбоку от прямой мышцы живота (в норме 1,5-2 см). Подлопаточная складка — в норме ее толщина не должна превышать 2 см, складка на животе у мужчин в норме до 1-2 см, у женщин — до 2-4 см.
В идеале для измерения использовать специальный прибор, называемый калипером [93], который сжимает складку кожи на определенных участках тела и через несколько секунд дает показатель толщины в миллиметрах. В реальной практике с помощью пальцев (большого и указательного или трех пальцев) захватывается кожно-жировая складка на выбранном участке тела перпендикулярно к поверхности кожи. Складка должна включать кожу и подкожный жир, не должна быть болезненной и не должна захватить только кожу или мышцы. Увеличение толщины кожной складки, особенно в области живота, может свидетельствовать о наличии избыточного веса или ожирения.
Индекс формы тела (ABSI) или индекс ожирения в области талии
Определяется по формуле: ИМТ2/3 × Рост1/2 и сильно коррелирует с МС, ССР и выраженностью абдоминального ожирения [73].
Для более детальной оценки распределения и количественной оценки жировых отложений используются показатели ОТ, ОБ, ОТ/ОБ и методы денситометрии, биоимпедансометрии, ультразвукового исследования (УЗИ), компьютерной томографии (КТ), магнитно-резонансной томографии (МРТ), двухэнергетической рентгеновской абсорбциометрии (DXA), позитронно-эмиссионной томографии (ПЭТ).
Биоимпедансометрия
Метод не является эталонным [94], но широко используется для оценки композиционного состава тела. Доступность обусловлена низкой стоимостью и простотой методики. В основе лежит измерение электрического сопротивления (импеданса) тканей, имеющих различную проводимость при прохождении через них переменного тока. Это позволяет оценить количество жировой и мышечной массы, внутри- и внеклеточной жидкости. Метод позволяет проводить анализ измене- ния жировой мышечной массы, общей воды в организме в динамики, в т.ч. у детей, подростков и спортсменов [94-96].
Гидростатическая денситометрия и плетизмография с вытеснением воздуха
Гидростатическая денситометрия (метод подводного взвешивания) основана на различиях плотности жировой и мышечной ткани. С использованием специальных формул после взвешивания пациента и оценки остаточного объема легких рассчитывается плотность тела и процентное содержание жира в организме. Метод не может быть использован в рутинной клинической практике в связи с его техническими сложностями (длительность исследования ~1 ч) и необходимостью погружения тела пациента в воду [97].
При воздушной плетизмографии объем тела оценивается по разнице между объемом воздуха в пустой камере и объемом воздуха и изменением давления воздуха после того, как в нее помещается человек. Метод позволяет измерить общую плотность тела, общее количество жира в организме. Время измерения составляет 5-8 мин, но требует специального оборудования, что ограничивает его использование в рутинной практике [98].
DXA
Метод DXA, основанный на особенностях взаимодействия рентгеновского излучения с различными тканями (костной, жировой и мышечной), дает возможность определить индекс жировой и безжировой массы, соотношение жира туловище/конечности и минеральную костную плотность (что активно используется для диагностики остеопороза). Проведения DXA позволяет более точно диагностировать и классифицировать ожирение по сравнению со стандартными антропометрическими измерениями [99]. Метод позволяет более точно диагностировать саркопению у лиц пожилого возраста и пациентов с СД 2 типа [100]. Метод позволяет быстро получить результаты, но имеет достаточно высокую стоимость.
УЗИ
УЗИ позволяет оценить количество подкожной жировой ткани или висцеральной жировой ткани и массу скелетных мышц. Проводят измерение расстояния между передней стенкой аорты и задней поверхностью прямых мышц живота на уровне 5 см ниже мечевидного отростка с последующей оценкой толщины подкожной жировой ткани и висцеральной жировой ткани, определяемой на этом же уровне, а также их соотношения. Данные, полученные этим методом, прямо коррелирует с данными о мышечной массе, полученными с использованием наиболее точных методов DXA, КТ и МРТ, но УЗИ является гораздо более простым, доступным и экономичным методом диагностики [101].
КТ
Метод основан на вычислении коэффициента ослабления рентгеновского излучения, проходящего через различные ткани тела человека. Для определения объема подкожной жировой ткани и висцеральной жировой ткани делают срезы на уровне поясничного отдела позвоночника (L1-L3), т.к. считается, что именно в этой области распределение жировой ткани наиболее точно коррелирует с ее распределением во всем организме [101]. Наряду с МРТ, метод считается "золотым стандартом" при оценке распределения и композиции жировой и мышечной тканей.
МРТ
Преимуществами метода являются высокая степень визуализации мягких тканей в сочетании с его доступностью и возможностью использования при более частом динамическом наблюдении в связи с отсутствием воздействия ионизирующим излучением. Метод позволяет различать и количественно оценить подкожно-жировую клетчатку и висцеральные жировые отложения в т.ч. в паренхиматозных органах [102].
Измерение площади сечения подкожной жировой клетчатки на уровне L3-L4 наиболее точно коррелирует с подкожной клетчаткой всего тела. Наиболее точно отражают количество абдоминальной жировой ткани срезы, проведенные на уровне 5-10 см выше L4-L5 или на уровне T12-L1 [103].
Выполнение МРТ на уровне поясничного отдела позвоночника позволяет дополнительно выявить пациентов с признаками ожирения по увеличению площади висцеральной жировой ткани, даже если они имели нормальные показатели ИМТ [104].
ПЭТ
Для проведения ПЭТ чаще всего используется 18F-фтордезоксиглюкоза, которая накапливается в тканях с высоким метаболизмом глюкозы. Ее накопление отслеживается при помощи ПЭТ-сканирования, в т.ч. в сочетании с КТ. Преимуществом ПЭТ является возможность оценки депо бурой жировой ткани (оценка ее плотности и метаболической активности), играющей важную роль в патогенезе ожирения. Сочетание ПЭТ и МРТ дает возможность более точно оценить объем бурой жировой ткани в конкретной области [105]. Недостатком использования ПЭТ является высокая стоимость.
При обследовании пациента с ИзМТ и ожирении необходимо обращать внимание на высокую частоту ассоциированных с ними заболеваний:
- пульмонологических — ОСА [106], бронхиальной астмы, синдрома Пиквика (гиповентиляция) [107] и предрасположенностью к респираторным инфекциям;
- кардиологических — ИБС [108], артериальной и легочной гипертензии, сердечной недостаточности с сохраненной фракцией выброса, кардиомиопатии;
- неврологических — инсульт/транзиторная ишемическая атака, идиопатической внутричерепной гипертензии, парестетической мералгии;
- гастроэнтерологических — патологии желчного пузыря (холецистит, холелитиаз), метаболически ассоциированная жировая болезнь печени, гастро-эзофагеальная рефлюксная болезнь;
- эндокринных — СД 2 типа;
- гинекологических — снижение фертильности, расстройства менструального цикла, гиперандрогения, поликистоз яичников;
- урологических — гипогонадизм (мужчины), стрес- совое недержание мочи;
- кожных — acanthosis nigricans, гирсутизм (женщины), целлюлит, опрелости, склонность к бактериальным и грибковым инфекциям;
- венозной системы — варикоз, лимфостаз, тромбоз глубоких вен;
- опорно-двигательной системы — остеоартрит, хроническая люмбалгия, варусная деформация шейки бедренной кости, смещение эпифиза головки бедренной кости, болезнь Блаунта (нарушение роста костей в области колена, приводящее к искривлению голени), болезнь Легга-Кальве-Пертеса (головка бедренной кости временно теряет кровоснабжение, что приводит к разрушению кости и воспалению);
- психических — депрессия, стигматизация.
Кроме этого, при обследовании пациента с ИзМТ и ожирением необходимо иметь повышенную онконастороженность в отношении как минимум 13 ви- дов новообразований, тесно ассоциированных с ними, которые обуславливают до 40% от всех случаев онкологических заболеваний. Имеются определённые гендерные различия в предрасположенности к конкретным формам новообразований при ожирении. Так, у мужчин повышен риск новообразований прямой кишки, предстательной железы, меланомы, а у женщин — эндометрия, желчного пузыря, молочной железы, поджелудочной железы, у обоих полов — пищевода, кишечника, почки, щитовидной железы, неходжинской лимфомы, миеломы [109][110]2.
Особенности лечения
Основными целями лечения являются предотвращение дальнейшего увеличения массы тела и развития осложнений путем поддержания метаболического здоровья пациента, и лечение сопутствующих заболеваний, если они уже имеются [111]. Снижение массы висцерального жира лежит в основе эффективной терапии. Показано, что для уменьшения площади висцерального жира на 1 см2 необходимо снизить массу тела на 4,7-6 кг [112]. Наличие или отсутствие сопутствующих заболеваний, течение которых напрямую ассоциировано с ИзМТ (СД 2 типа, неалкогольная жировая болезнь печени (НАЖБП), синдром обструктивного апноэ сна и т.д.), и их тяжесть определяют выбор терапии. У пациентов с ИзМТ и предиабетом рекомендовано снижение массы тела не менее 10% от исходной с целью профилактики развития СД 2 типа [113-115]. У пациентов с ИзМТ и СД 2 типа рекомендовано снижение массы тела не менее 5-15% от исходной, с целью скорейшего достижения целевого гликированного гемоглобина и/или снижения доз гипогликемических препаратов [113-116].
Пациентам с ИзМТ при ИМТ ≥27 кг/м2 при наличии факторов риска и/или коморбидных заболеваний может быть рекомендовано назначение медикаментозной терапии [117][118]. Снижение массы тела <3% у пациентов с СД и <5% у пациентов без СД от исходного или отсутствие уменьшения ОТ через 3 мес. медикаментозного лечения считается критерием неэффективности терапии. В качестве медикаментозной терапии ИзМТ в Российской Федерации могут использоваться следующие лекарственные препараты: тирзепатид, семаглутид, лираглутид, орлистат, сибутрамин.
Агонисты рецепторов человеческого ГПП-1 (арГПП-1) (семаглутид и лираглутид) и агонист рецепторов ГИП и ГПП-1 (арГПП-1/ГИП) (тирзепатид) регулируют аппетит с помощью усиления чувства наполнения желудка и насыщения, одновременно ослабляя чувство голода и уменьшая предполагаемое потребление пищи.
Тирзепатид показан к применению у взрослых в качестве дополнительной терапии при соблюдении диеты с пониженным содержанием калорий и увеличении физической активности для контроля массы тела, включая снижение и поддержание массы тела у взрослых людей с исходным ИМТ: >30 кг/м2 (ожирение); или >27 кг/м2 до <30 кг/м2 при наличии как минимум одного связанного с избыточным весом сопутствующего заболевания (например, артериальная гипертензия (АГ), дислипидемия, ОСА, ССЗ, предиабет или СД 2 типа). Начальная доза составляет 2,5 мг подкожно 1 раз в нед., с последующей стандартной титрацией (доза увеличивается каждые 4 нед. для улучшения желудочно-кишечной переносимости до достижения терапевтической — 10-15 мг в нед.).
Рандомизированное клиническое исследование SURMOUNT-1 показало, что применение тирзепатида у пациентов с ИзМТ и ожирением без СД 2 типа вызывает значительную дозозависимую потерю массы тела (-15,0% (95% доверительный интервал (ДИ): от -15,9 до -14,2) при еженедельных дозах тирзепатида 5 мг, -19,5% (95% ДИ: от -20,4 до -18,5) при дозах 10 мг и -20,9% (95% ДИ: от -21,8 до -19,9) при дозах 15 мг). Процент участников, у которых наблюдалось снижение веса на 5% или более, составил 85% (95% ДИ: 82-89), 89% (95% ДИ: 86-92) и 91% (95% ДИ: 88-94) при приеме 5 мг, 10 мг и 15 мг тирзепатида, соответственно, и 35% (95% ДИ: 30-39) при приеме плацебо. При этом 50% (95% ДИ: 46-54) и 57% (95% ДИ: 53-61) участников в группах 10 мг и 15 мг имели снижение веса тела на 20% или более по сравнению с 3% (95% ДИ: 1-5) в группе плацебо (P<0,001 для всех сравнений с плацебо) [119]. По сравнению с плацебо при применении тирзепатида наблюдалось значительное снижение ИМТ и ОТ, при этом средние значения составили -5,89 кг/м2 (от -8,97 до -2,81) и -12,31 см (от -13,93 до -10,68), соответственно [120]. При применении тирзепатида наблюдались улучшения всех кардиометаболических показателей (ОТ, систолическое и диастолическое АД, уровень инсулина натощак и уровень липидов).
Метаанализ сравнительных исследований тирзепатида и семаглутида показал, что средняя потеря массы тела составила -11,4% (от -15,3% до -8,27%) и -7,3% (от -8,3% до -6,08%), соответственно, средняя разница составила -4,84 кг (95% ДИ: от -6,21 до -3,47) в пользу тирзепатида [121].
У пациентов с СД 2 типа тирзепатид продемонстрировал положительное влияние на АД, СРБ и липидный профиль [122], а также значительное снижение уровня гликированного гемоглобина и улучшение гликемического контроля [123].
По данным ретроспективного когортного исследования, включившего данные о терапии 140308 пациентов с СД 2 типа [124], лечение тирзепатидом в сравнении с применением арГПП-1 было связано с более низкими рисками смертности от всех причин (скорректированное отношение рисков (скорОР) 0,58; 95% ДИ: 0,45-0,75), крупных неблагоприятных сердечно-сосудистых событий (скорОР 0,80; 95% ДИ: 0,71-0,91), совокупностью крупных неблагоприятных сердечно-сосудистых событий и смертности от всех причин (скорОР 0,76; 95% ДИ: 0,68-0,84), почечными событиями (скорОР 0,52; 95% ДИ: 0,37-0,73), острым повреждением почек (скорОР 0,78; 95% ДИ: 0,70-0,88) и серьезными нежелательными явлениями со стороны почек (скорОР 0,54; 95% ДИ: 0,44-0,67).
В исследовании SUMMIT у пациентов с СНсФВ и ожирением тирзепатид снижал сердечно-сосудистую смертность и количество госпитализаций [125]. Лечение тирзепатидом приводило к снижению систолического АД (предполагаемая разница в лечении (ETD) -5 мм рт.ст., 95% ДИ: от -7 до -3; P<0,001), уменьшало предполагаемый объем крови (ETD -0,58 л, 95% ДИ: от -0,63 до -0,52; P<0,001) и снижало уровни СРБ (ETD -37,2%, 95% ДИ: от -45,7 до -27,3; P<0,001). Эти изменения сопровождались увеличением расчетной скорости клубочковой фильтрации (ETD 2,90 мл/мин/1,73 м2 год 1, 95% ДИ: 0,94-4,86; P=0,004), уменьшением соотношения альбумина и креатинина в моче (ETD 24 нед., -25,0%, 95% ДИ: от -36 до -13%; P<0,001; 52 нед., -15%, 95% ДИ: от -28 до 0,1; P=0,051), снижением уровня N-концевого промозгового натрийуретического пептида (ETD 52 нед., -10,5%, 95% ДИ: от -20,7 до 1,0%; P=0,07) и снижение уровня тропонина Т (ETD 52 нед., -10,4%, 95% ДИ: от -16,7 до -3,6; P=0,003) [126].
В исследовании 2 фазы SYNERGY-NASH у пациентов с НАЖБП и умеренным или тяжёлым фиброзом лечение тирзепатидом в течение 52 нед. было более эффективным, чем плацебо, в отношении разрешения НАЖБП без усугубления фиброза. Процент участников, у которых наблюдалось улучшение по крайней мере на одну стадию фиброза без ухудшения НАЖБП, составил 30% в группе плацебо, 55% в группе тирзепатида 5 мг, 51% в группе тирзепатида 10 мг и 51% в группе тирзепатида 15 мг [127].
Семаглутид показан к применению у взрослых в дополнение к низкокалорийной диете и физической нагрузке для контроля массы тела, включая снижение и поддержание массы тела, у взрослых с ИМТ ≥27 кг/м2 при наличии по крайней мере одной сопутствующей патологии, связанной с ИзМТ, например дисгликемии (предиабет или СД 2 типа), АГ, дислипидемии, синдром обструктивного апноэ сна или ССЗ. Начальная доза составляет 0,25 мг подкожно 1 раз в нед., с последующей стандартной титрацией (доза увеличивается каждые 4 нед. для улучшения желудочно-кишечной переносимости до достижения терапевтической — 2,4 мг в сут.).
Метаанализ применения подкожного семаглутида 2,4 мг у пациентов с ИзМТ и ожирением без СД 2 типа показал, что по сравнению с плацебо семаглутид вызвал значительную дозозависимую потерю массы тела (средняя разница (MD): -10,09%; 95% ДИ: от -11,84 до -8,33; p<0,00001), большее снижение ИМТ (MD: -3,71 кг/м2; 95% ДИ: от -4,33 до -3,09; p<0,00001) и ОТ (MD: -8,28 см; 95% ДИ: от -9,51 до -7,04; p<0,00001), а также приводил к потере веса более чем на 5, 10, 15 и 20% у большей части пациентов. Кроме того, семаглутид продемонстрировал положительное влияние на АД, СРБ и липидный профиль [128], а также конверсию предиабета в СД 2 типа [129]. Аналогичная эффективность была продемонстрирована в метаанализе, включавшем как пациентов с СД 2 типа, так и без него [130]. Семаглутид 2,4 мг и лираглутид 3,0 мг являются препаратами выбора для снижения массы тела у пациентов с ИМТ ≥27 кг/м2 в сочетании с АГ [128, 131-133]. Применение семаглутида у пациентов с ИМТ ≥27 кг/м2 и НАЖБП ассоциировано со снижением уровней аланинаминотрансферазы (MD: 14,07 Ед/л (95% ДИ: от 19,39 до -8,75); p<0,001) и аспартатаминотрансферазы (MD: 6,89 Ед/л (95% ДИ: от 9,14 до -4,63); p<0,001). Также отмечено значительное снижение содержания жира в печени (MD: 4,97% (95% ДИ: от 6,65 до -3,29); p<0,001) и индекса фиброза печени (MD: 0,96 кПа (95% ДИ: от 1,87 до -0,04); p=0,04) [134].
арГПП-1, вероятно, оказывают незначительное влияние на течение ХБП и комплексные исходы со стороны почек [135].
Лираглутид — рекомендуется пациентам с ИМТ ≥27 кг/м2 при наличии факторов риска и/или коморбидных заболеваний; начальная доза составляет 0,6 мг подкожно 1 раз в сут., с последующей стандартной титрацией (доза увеличивается на 0,6 мг с интервалами не менее 1 нед. для улучшения желудочно-кишечной переносимости до достижения терапевтической — 3,0 мг в сут.) [136-138]. Лираглутид 3,0 мг обеспечивает эффективную и устойчивую потерю массы тела [139], однако менее выраженную, чем семаглутид 2,4 мг (по данным метаанализа, применение семаглутида 2,4 мг приводило к средней потере массы тела на 12,47 кг, лираглутида 3,0 мг -5,24 кг [140], приводя к снижению массы тела более, чем на 5% и 10% в 65,3% и 30,7% случаев для лираглутида 3 мг и 86,6% и 75,3% для семаглутида 2,4 мг, соответственно [141]).
Лираглутид 3,0 мг также положительно влияет на динамику кардиометаболических факторов риска на фоне снижения массы тела [131] и может рассматриваться как один из предпочтительных вариантов для пациентов с ИзМТ и наличием сопутствующих ССЗ в связи с доказанным [132][133] снижением ССР, значительным снижением риска развития СД 2 типа [142] и благоприятным профилем безопасности и переносимости. Лираглутид 3,0 мг положительно влияет на АД у пациентов с ИМТ ≥27 кг/м2 в сочетании с АГ [131-133], однако следует тщательно контролировать частоту сердечных сокращений (ЧСС) у пациентов, получающих лираглутид [136-138]. Для лираглутида 3,0 мг было показано снижение содержания жировой ткани в печени у пациентов с СД 2 типа и НАЖБП, однако влияние на печеночные ферменты в различных исследованиях оказалось нейтральным или слабоположительным [143-145].
Стоит отметить, что после прекращения терапии арГПП-1/ГИП восстановление массы тела пропорционально ее исходной потере. Пациенты, принимавшие лираглутид, набрали в среднем 2,20 кг (95% ДИ: 1,69-2,70, P<0,00001), пациенты, принимавшие семаглутид/тирзепатид, набрали 9,69 кг (95% ДИ: 5,78-13,60, P<0,00001) [146].
арГПП-1/ГИП противопоказаны при медуллярном раке щитовидной железы в анамнезе, в т.ч. семейном, множественной эндокринной неоплазии II ти- па, тяжелой депрессии, суицидальных мыслях или поведении, в т.ч. в анамнезе, почечной и печеночной недостаточности тяжелой степени, хронической сердечной недостаточности IV функционального класса (в соответствии с классификацией NYHA), у пациентов в возрасте ≥75 лет. Применение арГПП-1 у пациентов с воспалительными заболеваниями кишечника и диабетическим парезом желудка не рекомендуется, поскольку оно связано с транзиторными нежелательными реакциями со стороны ЖКТ, включая тошноту, рвоту и диарею. С осторожностью препараты применяют у пациентов с печеночной недостаточностью легкой и средней степени тяжести, заболеваниями щитовидной железы и наличием острого панкреатита в анамнезе.
Орлистат — препарат для лечения ожирения периферического действия, рекомендуется пациентам с ИМТ ≥28 кг/м2 при наличии коморбидных заболеваний в дозе 120 мг 3 раза в сут. во время еды или не позже 1 ч после приема пищи для снижения массы тела. Разрешенная максимальная длительность непрерывного лечения составляет 4 года [117][147][148]. Орлистат, будучи специфическим, длительно действующим ингибитором желудочно-кишечной липазы, оказывает терапевтический эффект в пределах ЖКТ и не обладает системными эффектами: препятствует расщеплению и последующему всасыванию жиров, поступающих с пищей (~30%), создавая тем самым дефицит энергии, что приводит к снижению массы тела в среднем на 3,06% (95% ДИ: 3,45-2,67) [118] или -3,07 кг (95% ДИ: от -3,76 до -2,37) [149]. Орлистат способствует также небольшому снижению общего холестерина, липопротеинов низкой плотности, триглицеридов и липопротеинов высокой плотности [150], причем независимо от степени снижения массы тела. Если прием пищи не состоялся или пища не содержит жира, то прием препарата можно пропустить. Применение орлистата у больных с ИзМТ позволяет существенно улучшить профиль факторов риска СД 2 типа, ССЗ и других заболеваний, ассоциированных с ИзМТ, что может благоприятно влиять на прогноз жизни у этой категории больных. Данных, позволяющих судить о влиянии орлистата на общую смертность или смертность от ССЗ, в настоящее время нет. Важным преимуществом препарата является его периферическое действие только в пределах ЖКТ и отсутствие системных эффектов. Орлистат противопоказан при острых панкреатитах и заболеваниях, сопровождающихся диареей, синдромом хронической мальабсорбции, холестазом. Орлистат повышает вероятность образования камней в желчном пузыре, однако рациональное потребление жиров не приводит к снижению моторики желчного пузыря. С учетом механизма действия, к числу побочных эффектов препарата относятся жирный стул, маслянистые выделения из прямой кишки, императивные позывы на дефекацию, учащение дефекации и недержание кала, боли в животе, повышенный метеоризм с некоторым количеством отделяемого. Выраженность и продолжительность побочных эффектов напрямую зависят от приверженности пациентов лечению и соблюдения рекомендаций по ограничению жиров в пище. Если рекомендованы поливитамины, их следует принимать не ранее чем через 2 ч после приема орлистата или перед сном.
Сибутрамин, сибутрамин + микрокристаллическая целлюлоза — препарат для лечения алиментарного ожирения с ИМТ 27 кг/м2 и более в сочетании с СД 2 типа и дислипидемией. Однако его негативное влияние на АД, ЧСС и риск развития сердечно-сосудистых осложнений делает его применение малоцелесообразным у большинства пациентов с ИзМТ, т.к. они уже имеют высокий риск развития сердечно-сосудистых осложнений [151]. Разрешенная максимальная длительность лечения составляет 1 год [117][152][153]. Лечение сибутрамином требует обязательного врачебного наблюдения. Контроль АД и пульса необходим у всех больных до начала лечения, далее с 1-го по 3-й мес. лечения — каждые 2 нед., с 4-го по 6-й мес. — ежемесячно, с 6-го по 12-й мес. — каждые 3 мес. Препарат отменяют при выявлении увеличения ЧСС в покое ≥10 уд./мин и/или повышении АД более чем на 10 мм рт.ст. во время двух визитов подряд, а также в случае, если оно при двух повторных измерениях превышает 140/90 мм рт.ст. при ранее компенсированной АГ. Препарат не может быть назначен пациентам с неконтролируемой АГ, ИБС, декомпенсацией хронической сердечной недостаточности, нарушением ритма сердца, цереброваскулярными заболеваниями (инсультом, транзиторными нарушениями мозгового кровообращения), окклюзионными заболеваниями периферических артерий, в возрасте старше 65 лет, при тяжелых поражениях печени и почек, которые могут встречаться при ожирении, в случае одновременного приема или спустя <2 нед. после отмены ингибиторов моноаминоксидазы или других препаратов, действующих на центральную нервную систему (в т.ч. антидепрессантов), при серь- езных нарушениях питания и психических заболеваниях, тиреотоксикозе, феохромоцитоме, закрытоугольной глаукоме, доброкачественной гиперплазии предстательной железы.
Алгоритмы фармакотерапии ИзМТ и выбора препаратов представлены в таблицах 1, 2 и рисунках 1, 2.
Таблица 1
Выбор препаратов для фармакотерапии ИзМТ в зависимости от коморбидной патологии [154]
Тирзепатид/Семаглутид/Лираглутид | Орлистат | Сибутрамин, Сибутрамин + МКЦ, Сибутрамин + метформин | |
Артериальная гипертензия | + | + | - |
ИБС, цереброваскулярная болезнь | + | + | - |
Хроническая сердечная недостаточность | + | - | |
Хроническая болезнь почек | + | - | |
Предиабет/сахарный диабет 2 типа | + | - | |
МАЖБП | + | + | - |
Панкреатиты | +/- | + | + |
Медуллярный рак щитовидной железы | - | + | + |
Желчнокаменная болезнь | +/- | +/- | + |
Холестаз | + | - | + |
Заболевания ЖКТ, сопровождающиеся диареей | +/- | - | + |
Сокращения: ЖКТ — желудочно-кишечный тракт, ИБС — ишемическая болезнь сердца, МКЦ — микрокристаллическая целлюлоза, МАЖБП — метаболически ассоциированная жировая болезнь печени.
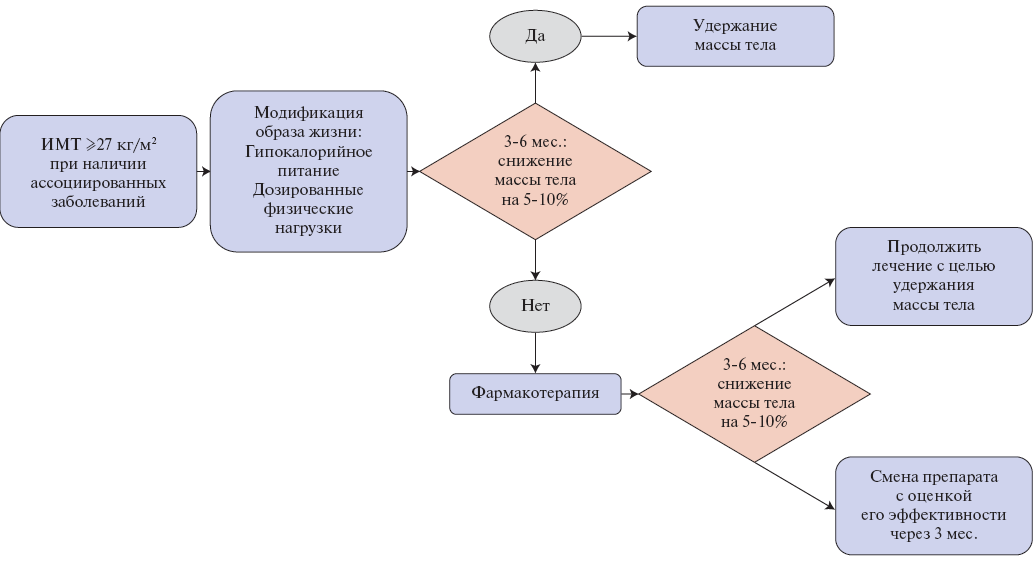
Рис. 1. Алгоритм медикаментозной терапии ИзМТ [154].
Сокращение: ИМТ — индекс массы тела.
Таблица 2
ССР и преимущества препаратов, используемых для снижения веса [155]
Препарат | Комплексные СС исходы | Смерть от СС заболеваний | Нефатальный ИМ | Нефатальный инсульт | САД | ДАД | ЧСС | ЛВП | ЛНП | ТГ | СН | вч-СРБ [156] |
Сибутрамин [157] | ↑↑ | – | ↑↑ | ↑↑ | ↑↑* | ↑↑* | ↑↑ | НД | НД | НД | НД | НД |
Орлистат | НД | НД | НД | НД | ↓ | ↓ | НД | ↑ | – | – | НД | ↓ |
Лираглутид 1,8 мг при СД 2 типа | ↓↓ | ↓↓ | ↓ | ↓ | ↓↓ | ↑↑ | ↑↑ | – | – | – | ↓ | ↓↓ |
Лираглутид 3,0 мг для лечения ожирения | – [132] | НД [132] | НД [132] | НД [132] | ↓↓ | ↓↓ | ↑↑ | ↑↑ | ↓↓ | ↓↓ | НД | ↓↓ |
Семаглутид | ↓↓ | ↓ | ↓ | ↓↓ | ↓↓ | – | ↓↓ | ↓↓ | ↑↑ | ↑↑ | ↓ | ↓↓ [158] |
Тирзепатид | ожидаются результаты РКИ при ожирении и СД 2 типа ↓↓ при СД 2 типа СНнФВ с ожирением [125] | ожидаются результаты РКИ при ожирении и СД 2 типа ↓↓ при СД 2 типа СНнФВ с ожирением [125] | ожидаются результаты РКИ при ожирении и СД 2 типа | ожидаются результаты РКИ при ожирении и СД 2 типа | ↓↓ [119] | ↓↓ [119] | ↑↑ [119] | ↓↓ [119] | ↓↓ [119] | ↓↓ при СД 2 типа СНнФВ с ожирением [125] | ↓↓ [159] | |
Эксенатид при СД 2 типа | – | ↓ | – | ↓ | ↓↓ | ↑↑ | ↑↑ | НД | ↓↓ | ↓↓ | ↓ | ↓↓ [160] |
Дулагутид при СД 2 типа | ↓↓ | ↓ | ↓ | ↓↓ | ↓↓ | – | ↑↑ | ↓↓ | НД | НД | ↓↓ | ↓↓ [161] |
Ликсисенатид при СД 2 типа | ↑ | ↓ | ↑ | ↑ | ↓↓ | НД | ↑↑ | НД | НД | НД | ↓ | НД |
Метформин при СД 2 типа | ↓ | ↓ | ↓ | ↓↓ | НД | НД | НД | НД | ↓↓ | ↓↓ | НД | ↓↓ [161] |
Ингибиторы НГЛТ-2 при СД 2 типа | ↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | – | – | – | – | ↓ | ↓↓ [161] |
Примечание: * — в РКИ SCOUT АД в группе сибутрамина снижалось в сравнении с исходным, но было выше, чем в группе плацебо: средние различия САД и ДАД между группами колебались от -0,3 до 1,2 мм рт.ст. и от 0,6 до 1,4 мм рт.ст., соответственно; ↓↓ — статистически значимое снижение; ↑↑ — статистически значимое повышение; ↓ или ↑ — незначимое изменение; "–" — указано/нет изменений; НД — данные не представлены.
Сокращения: вч-СРБ — высокочувствительный С-реактивный белок, ДАД — диастолическое артериальное давление, ИМ — инфаркт миокарда, ЛВП — липопротеины высокой плотности, ЛНП — липопротеины низкой плотности, НГЛТ-2 — натрий-глюкозный котранспортер 2 типа, РКИ — рандомизированные клинические исследования, САД — систолическое артериальное давление, СД — сахарный диабет, СН — сердечная недостаточность, СНнФВ — сердечная недостаточность с низкой фракцией выброса, СС — сердечно-сосудистый, ТГ — триглицериды, ЧСС — частота сердечных сокращений.
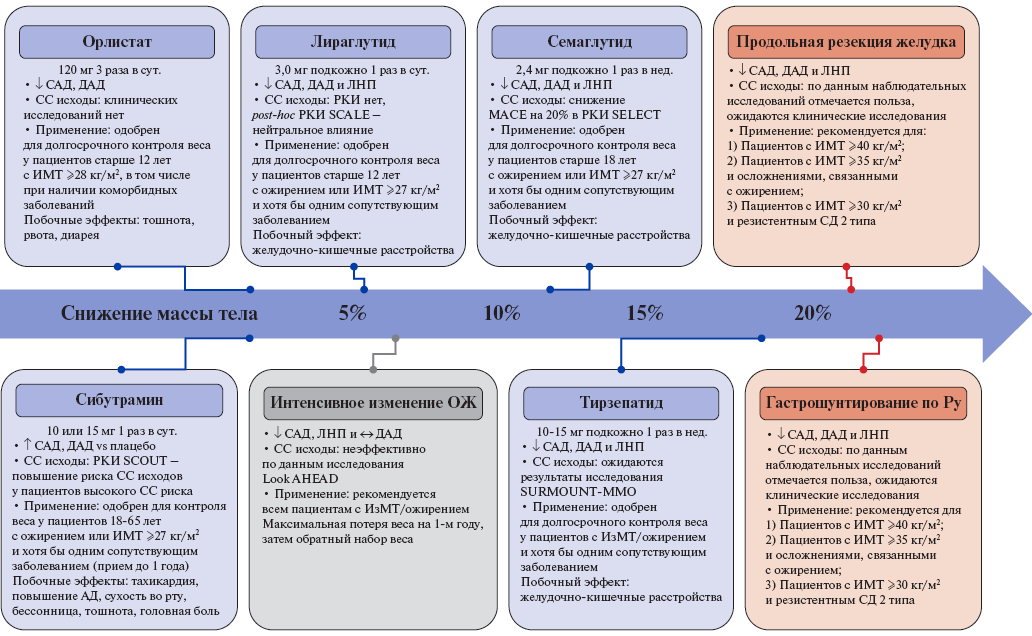
Рис. 2. Текущие данные о влиянии каждого вида вмешательства при ожирении на массу тела, факторы риска ССЗ и сердечно-сосудистые исходы, а также показания к применению этих вмешательств [162].
Сокращения: АД — артериальное давление, ДАД — диастолическое артериальное давление, ИзМТ — избыточная масса тела, ИМТ — индекс массы тела, ЛНП — липопротеины низкой плотности, ОЖ — ожирение, РКИ — рандомизированные клинические исследования, САД — систолическое артериальное давление, СД — сахарный диабет, СС — сердечно-сосудистый, MACE — крупные сердечно-сосудистые события.
1 Клинические рекомендации. Ожирение. 2024. https://cr.minzdrav.gov.ru/preview-cr/28_3.
2 National Cancer Institute. Obesity and Cancer. NCI. Available at https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet. Reviewed January 28, 2025; Accessed: May 23, 2025.
Список литературы
1. Danpanichkul P, Suparan K, Kim, D, Wijarnpreecha K. What Is New in Metabolic Dysfunction-Associated Steatotic Liver Disease in Lean Individuals: From Bench to Bedside. J. Clin. Med. 2024;13:278. doi:10.3390/jcm13010278.
2. Mukhopadhyay P, Ghosh S, Bhattacharjee K, et al. Lean Metabolic Syndrome: A Concept or a Reality? Indian J Endocrinol Metab. 2018;22(3):303-7. doi:10.4103/ijem.IJEM_639_17. Erratum in: Indian J Endocrinol Metab. 2018;22(6):868. doi:10.4103/2230-8210.246859.
3. Tang A, Ng CH, Phang PH, et al. Comparative Burden of Metabolic Dysfunction in Lean NAFLD vs Non-lean NAFLD — A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21(7):1750-60.e12. doi:10.1016/j.cgh.2022.06.029.
4. Wang W, Ren J, Zhou W, et al. Lean non-alcoholic fatty liver disease (Lean-NAFLD) and the development of metabolic syndrome: a retrospective study. Sci Rep. 2022;12(1):10977. doi:10.1038/s41598-022-14701-0.
5. Osadnik K, Osadnik T, Gierlotka M, et al. Metabolic syndrome is associated with similar long-term prognosis in those living with and without obesity: an analysis of 45 615 patients from the nationwide LIPIDOGRAM 2004-2015 studies. Eur J Prev Cardiol. 2023;30(12):1195-204. doi:10.1093/eurjpc/zwad101.
6. Sanyal D. Lean Metabolic Syndrome: An Emerging Concept. Indian J Endocrinol Metab. 2018;22(3):301-2. doi:10.4103/2230-8210.236782.
7. Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826-37. doi:10.1016/S0140-6736(11)60812-X.
8. González-Muniesa P, Mártinez-González M-A, Hu FB, et al. Obesity. Nat. Rev. Dis. Prim. 2017;3:17034. doi:10.1038/nrdp.2017.34.
9. Bray MS, Loos R, McCaffery J, Ling C, et al. The The Conference Working Group NIH working group report–using genomic information to guide weight management: From universal to precision treatment. Obesity. 2016;24:14-22. doi:10.1002/oby.21381.
10. Elks CE, Hoed MD, Zhao JH, et al. Variability in the Heritability of Body Mass Index: A Systematic Review and Meta-Regression. Front. Endocrinol. 2012;3:29. doi:10.3389/fendo.2012.00029.
11. Winkler TW, Justice AE, Graff M, et al. The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study. PLoS Genet. 2015;11:e1005378. doi:10.1371/journal.pgen.1005378.
12. Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197-206. doi:10.1038/nature14177.
13. Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1940;78:149-72. doi:10.1002/ar.1090780203.
14. Williams DM, Nawaz A, Evans M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020;11:1199-216. doi:10.1007/s13300-020-00816-y.
15. Yamamoto H, Kishi T, Lee CE, et al. Glucagon-Like Peptide-1-Responsive Catecholamine Neurons in the Area Postrema Link Peripheral Glucagon-Like Peptide-1 with Central Autonomic Control Sites. J. Neurosci. 2003;23:2939-46. doi:10.1523/JNEUROSCI.23-07-02939.2003.
16. Morton G, Schwartz M. The NPY/AgRP neuron and energy homeostasis. Int. J. Obes. 2001;25:S56-S62. doi:10.1038/sj.ijo.0801915.
17. Badman MK. The Gut and Energy Balance: Visceral Allies in the Obesity Wars. Science. 2005;307:1909-14. doi:10.1126/science.1109951.
18. Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007;13:803-11. doi:10.1038/nm1611.
19. Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 1996;81:3419-23. doi:10.1210/jcem.81.9.8784108.
20. Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011;121:1424-8. doi:10.1172/JCI46229.
21. Chen Y, Lin Y-C, Zimmerman C, et al. Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife. 2016;5:e18640. doi:10.7554/eLife.18640.
22. Betley JN, Xu S, Cao ZFH, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nat. Cell Biol. 2015;521:180-5. doi:10.1038/nature14416.
23. Cummings DE, Purnell JQ, Frayo RS, et al. A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes. 2001;50:1714-9. doi:10.2337/diabetes.50.8.1714.
24. Ravussin E, Smith SR, Mitchell JA, et al. Enhanced Weight Loss With Pramlintide/ Metreleptin: An Integrated Neurohormonal Approach to Obesity Pharmacotherapy. Obesity. 2009;17:1736-43. doi:10.1038/oby.2009.184.
25. Müller T, Nogueiras R, Andermann M, et al. Ghrelin. Mol. Metab. 2015;4:437-60. doi:10.1016/j.molmet.2015.03.005.
26. Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nat. Cell Biol. 2001;409:194-8. doi:10.1038/35051587.
27. Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-60. doi:10.1038/45230.
28. Wang W, Tao Y-X. Progress in Molecular Biology and Translational Science. Volume 140. Elsevier BV; Amsterdam, The Netherlands: 2016. Ghrelin Receptor Mutations and Human Obesity; pp. 131-50.
29. Holst B. Ghrelin receptor mutations — too little height and too much hunger. J. Clin. Investig. 2006;116:637-41. doi:10.1172/JCI27999.
30. Sun Y, Ahmed S, Smith RG. Deletion of Ghrelin Impairs neither Growth nor Appetite. Mol. Cell. Biol. 2003;23:7973-81. doi:10.1128/MCB.23.22.7973-7981.2003.
31. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA. 2004;101:4679-84. doi:10.1073/pnas.0305930101.
32. Cummings DE, Weigle DS, Frayo RS, et al. Plasma Ghrelin Levels after Diet-Induced Weight Loss or Gastric Bypass Surgery. N. Engl. J. Med. 2002;346:1623-30. doi:10.1056/NEJMoa012908.
33. Neary NM, Small CJ, Wren AM, et al. Ghrelin Increases Energy Intake in Cancer Patients with Impaired Appetite: Acute, Randomized, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2004;89:2832-6. doi:10.1210/jc.2003-031768.
34. Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005;16:297-313. doi:10.1097/00008877-200509000-00004.
35. Pertwee RG. Pharmacological Actions of Cannabinoids. Handb. Exp. Pharmacol. 2005;168:1-51. doi:10.1007/3-540-26573-2_1.
36. Richey JM, Woolcott O. Revisiting the Endocannabinoid System and Its Therapeutic Potential in Obesity and Associated Diseases. Curr. Diabetes Rep. 2017;17:99. doi:10.1007/s11892-017-0924-x.
37. Sekar R, Wang L, Chow BKC. Central Control of Feeding Behavior by the Secretin, PACAP, and Glucagon Family of Peptides. Front. Endocrinol. 2017;8:18. doi:10.3389/fendo.2017.00018.
38. Li Y, Schnabl K, Gabler S-M, et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell. 2018;175:1561-74.e12. doi:10.1016/j.cell.2018.10.016.
39. Johnson LR. Gastrointestinal Phisiology. 9th ed. Elsevier; Philadelphia, PA, USA: 2019.
40. Muurahainen N, Kissileff HR, Derogatis AJ, Pi-Sunyer FX. Effects of cholecystokinin-octapeptide (CCK-8) on food intake and gastric emptying in man. Physiol. Behav. 1988;44:645-9. doi:10.1016/0031-9384(88)90330-7.
41. Grill HJ, Hayes MR. Hindbrain Neurons as an Essential Hub in the Neuroanatomically Distributed Control of Energy Balance. Cell Metab. 2012;16:296-309. doi:10.1016/j.cmet.2012.06.015.
42. Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 2016;7:11905. doi:10.1038/ncomms11905.
43. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017;38:267-96. doi:10.1210/er.2017-00111.
44. Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018;20((Suppl. 1)):5-21. doi:10.1111/dom.13129.
45. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993;91:301-7. doi:10.1172/JCI116186.
46. Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585-96. doi:10.1210/endo.136.8.7628397.
47. Butler PC, Chou J, Carter WB, et al. Effects of Meal Ingestion on Plasma Amylin Concentration in NIDDM and Nondiabetic Humans. Diabetes. 1990;39:752-6. doi:10.2337/diab.39.6.752.
48. Li Z, Kelly L, Gergi I, et al. Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell Metab. 2015;22:1059-67. doi:10.1016/j.cmet.2015.10.012.
49. Mietlicki-Baase EG, Reiner DJ, Cone J, et al. Amylin Modulates the Mesolimbic Dopamine System to Control Energy Balance. Neuropsychopharmacology. 2015;40:372-85. doi:10.1038/npp.2014.180.
50. Whiting L, McCutcheon J, Boyle CN, et al. The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc). Physiol. Behav. 2017;176:9-16. doi:10.1016/j.physbeh.2017.03.023.
51. Scherer PE, Williams S, Fogliano M, et al. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995;270:26746-9. doi:10.1074/jbc.270.45.26746.
52. Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017;18:1321. doi:10.3390/ijms18061321.
53. Fisman EZ, Tenenbaum A. Adiponectin: A manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc. Diabetol. 2014;13:103. doi:10.1186/1475-2840-13-103.
54. Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004;10:524-9. doi:10.1038/nm1029.
55. Hivert M-F, Sullivan L, Fox CS, et al. Associations of Adiponectin, Resistin, and Tumor Necrosis Factor-α with Insulin Resistance. J. Clin. Endocrinol. Metab. 2008;93:3165-72. doi:10.1210/jc.2008-0425.
56. Zaidi SI, Shirwany TA. Relationship of serum resistin with insulin resistance and obesity. J Ayub Med Coll Abbottabad. 2015;27(3):552-5.
57. Su K-Z, Li Y-R, Zhang D, et al. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front. Physiol. 2019;10:1399. doi:10.3389/fphys.2019.01399.
58. Recinella L, Orlando G, Ferrante C, et al. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020;11:578966. doi:10.3389/fphys.2020.578966.
59. Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013;123:3404-8. doi:10.1172/JCI67803.
60. Van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009;360:1500-8. doi:10.1056/NEJMoa0808718.
61. Warwick PM, Busby R. Influence of mild cold on 24 h energy expenditure in ‘normally’ clothed adults. Br. J. Nutr. 1990;63:481-8. doi:10.1079/BJN19900135.
62. Planavila A, Redondo I, Hondares E, et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 2013;4:2019. doi:10.1038/ncomms3019.
63. Lee P, Brychta RJ, Linderman J, et al. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: Relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J. Clin. Endocrinol. Metab. 2013;98:E98-E102. doi:10.1210/jc.2012-3107.
64. Correa-Burrows P, Rogan J, Blanco E, et al. Resolving early obesity leads to a cardiometabolic profile within normal ranges at 23 years old in a two-decade prospective follow-up study. Sci Rep. 2021;11(1):18927. doi:10.1038/s41598-021-97683-9.
65. Gutin I. In BMI We Trust: Reframing the Body Mass Index as a Measure of Health. Soc Theory Health. 2018;16(3):256-71. doi:10.1057/s41285-017-0055-0.
66. Bray GA. Beyond BMI. Nutrients. 2023;15(10):2254. doi:10.3390/nu15102254.
67. Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34(5):791-9. doi:10.1038/ijo.2010.5.
68. Khanna D, Peltzer C, Kahar P, Parmar MS. Body Mass Index (BMI): A Screening Tool Analysis. Cureus. 2022;14(2):e22119. doi:10.7759/cureus.22119.
69. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452.
70. Ross R, Berentzen T, Bradshaw AJ, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev. 2008;9(4):312-25. doi:10.1111/j.1467-789X.2007.00411.x.
71. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177-89. doi:10.1038/s41574-019-0310-7.
72. Campana EMG, Brandão AA. Waist Circumference: A Parameter of Vascular Health. Arq Bras Cardiol. 2022;119(2):265-6. English, Portuguese. doi:10.36660/abc.20220508.
73. Jayedi A, Soltani S, Zargar MS, et al. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. 2020;370:m3324. doi:10.1136/bmj.m3324.
74. Bosomworth NJ. Normal-weight central obesity: Unique hazard of the toxic waist. Can Fam Physician. 2019;65(6):399-408.
75. Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 2018;21(5):360-5. doi:10.1097/MCO.0000000000000485.
76. Mawaddatina T, Budihastuti UR, Rahayu D. Waist circumference, hip circumference, arm span, and waist-to-hip ratio high risk of polycystic ovarian syndrome. Scott Med J. 2021;66(4):186-90. doi:10.1177/00369330211043206.
77. Parker ED, Pereira MA, Stevens J, Folsom AR. Association of hip circumference with incident diabetes and coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Epidemiol. 2009;169(7):837-47. doi:10.1093/aje/kwn395.
78. Lanfer A, Mehlig K, Heitmann BL, Lissner L. Does change in hip circumference predict cardiovascular disease and overall mortality in Danish and Swedish women? Obesity (Silver Spring). 2014;22(3):957-63. doi:10.1002/oby.20604.
79. Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring). 2011;19(5):1083-9. doi:10.1038/oby.2011.38.
80. Tur JA, Bibiloni MDM. Anthropometry, Body Composition and Resting Energy Expenditure in Human. Nutrients. 2019;11(8):1891. doi:10.3390/nu11081891.
81. Zamaninour N, Ansar H, Pazouki A, Kabir A. Relationship Between Modified Body Adiposity Index and A Body Shape Index with Biochemical Parameters in Bariatric Surgery Candidates. Obes Surg. 2020;30(3):901-9. doi:10.1007/s11695-019-04256-x.
82. Schulze MB, Thorand B, Fritsche A, et al. Body adiposity index, body fat content and incidence of type 2 diabetes. Diabetologia. 2012;55(6):1660-7. doi:10.1007/s00125-012-2499-z.
83. Chang H, Simonsick EM, Ferrucci L, Cooper JA. Validation study of the body adiposity index as a predictor of percent body fat in older individuals: findings from the BLSA. J Gerontol A Biol Sci Med Sci. 2014;69(9):1069-75. doi:10.1093/gerona/glt165.
84. Myint PK, Kwok CS, Luben RN, et al. Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart. 2014;100(20):1613-9. doi:10.1136/heartjnl-2014-305816.
85. Burton RF. The waist-hip ratio: a flawed index. Ann Hum Biol. 2020;47(7-8):629-31. doi:10.1080/03014460.2020.1820079.
86. Haufs MG, Zöllner YF. Waist-Hip Ratio More Appropriate Than Body Mass Index. Dtsch Arztebl Int. 2020;117(39):659. doi:10.3238/arztebl.2020.0659a.
87. Jayedi A, Soltani S, Motlagh SZ, et al. Anthropometric and adiposity indicators and risk of type 2 diabetes: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2022;376:e067516. doi:10.1136/bmj-2021-067516.
88. Cao Q, Yu S, Xiong W, et al. Waist-hip ratio as a predictor of myocardial infarction risk: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(30):e11639. doi:10.1097/MD.0000000000011639.
89. Wang Y, Mao L, Zhang X. Waist-hip ratio is an independent predictor of moderate-to-severe OSA in nonobese males: a cross-sectional study. BMC Pulm Med. 2022;22(1):151. doi:10.1186/s12890-022-01886-3.
90. Xu Y, Li X, Hu T, et al. Neck circumference as a potential indicator of pre-sarcopenic obesity in a cohort of community-based individuals. Clin Nutr. 2024;43(1):11-7. doi:10.1016/j.clnu.2023.11.006.
91. Luo Y, Ma X, Shen Y, et al. Neck circumference as an effective measure for identifying cardio-metabolic syndrome: a comparison with waist circumference. Endocrine. 2017;55(3):822-30. doi:10.1007/s12020-016-1151-y.
92. Padilha CM, Pescuma JMS, Rodrigues ALCC, et al. Neck circumference as a marker of body adiposity in young to middle-aged adults. Nutrition. 2022;93:111496. doi:10.1016/j.nut.2021.111496.
93. Fosbøl MØ, Zerahn B. Contemporary methods of body composition measurement. Clin Physiol Funct Imaging. 2015;35(2):81-97. doi:10.1111/cpf.12152.
94. Holmes CJ, Racette SB. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients. 2021;13(8):2493. doi:10.3390/nu13082493.
95. Coëffier M, El Machkouri M, L’Huillier C, et al. Accuracy of bioimpedance equations for measuring body composition in a cohort of 2134 patients with obesity. Clin Nutr. 2022;41(9):2013-24. doi:10.1016/j.clnu.2022.07.032.
96. Karchynskaya V, Kopcakova J, Klein D, et al. Is BMI a Valid Indicator of Overweight and Obesity for Adolescents? Int J Environ Res Public Health. 2020;17(13):4815. doi:10.3390/ijerph17134815.
97. Mazahery H, von Hurst PR, McKinlay CJD, et al. Air displacement plethysmography (pea pod) in full-term and pre-term infants: a comprehensive review of accuracy, reproducibility, and practical challenges. Matern Health Neonatol Perinatol. 2018;4:12. doi:10.1186/s40748-018-0079-z.
98. Kuriyan R, Thomas T, Ashok S, et al. A 4-compartment model based validation of air displacement plethysmography, dual energy X-ray absorptiometry, skinfold technique & bio-electrical impedance for measuring body fat in Indian adults. Indian J Med Res. 2014;139(5):700-7.
99. Golja P, Robič Pikel T, Zdešar Kotnik K, et al. Direct Comparison of (Anthropometric) Methods for the Assessment of Body Composition. Ann Nutr Metab. 2020;76(3):183-92. doi:10.1159/000508514.
100. Heymsfield SB, Ebbeling CB, Zheng J, et al. Multi-component molecular-level body composition reference methods: evolving concepts and future directions. Obes Rev. 2015;16(4):282-94. doi:10.1111/obr.12261.
101. Liu X, He M, Li Y. Adult obesity diagnostic tool: A narrative review. Medicine (Baltimore). 2024;103(17):e37946. doi:10.1097/MD.0000000000037946.
102. Nabasenja C, Barry K, Nelson T, et al. Imaging individuals with obesity. J Med Imaging Radiat Sci. 2022;53(2):291-304. doi:10.1016/j.jmir.2022.02.003.
103. Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between singleslice areas and total volume. Am J Clin Nutr. 2004;80(2):271-8. doi:10.1093/ajcn/80.2.271.
104. Thomas EL, Brynes AE, McCarthy J, et al. Visceral adipose tissue volume measured by single-slice MRI at the L4–L5 level is closely correlated with total visceral adipose tissue volume. International Journal of Obesity. 2002;26:535-8. doi:10.1038/sj.ijo.0801937.
105. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):143-9. doi:10.1097/MED.0b013e328337a81f.
106. Li C, Ford ES, Zhao G, et al. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005-2006. Prev Med. 2010;51(1):18-23. doi:10.1016/j.ypmed.2010.03.016.
107. Ghimire P, Sankari A, Antoine MH, et al. Obesity-Hypoventilation Syndrome. [Updated 2025 Jun 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542216/.
108. Oreopoulos A, Padwal R, McAlister FA, et al. Association between obesity and health-related quality of life in patients with coronary artery disease. Int J Obes (Lond). 2010;34(9):1434-41. doi:10.1038/ijo.2010.73.
109. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi:10.1136/bmj.j477.
110. Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569-78. doi:10.1016/S0140-6736(08)60269-X.
111. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi:10.1186/1471-2458-9-88.
112. Rao S, Pandey A, Garg S, et al. Effect of Exercise and Pharmacological Interventions on Visceral Adiposity: A Systematic Review and Meta-analysis of Long-term Randomized Controlled Trials. Mayo Clin Proc. 2019;94(2):211-24. doi:10.1016/j.mayocp.2018.09.019.
113. Ryan DH, Yockey SR. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr Obes Rep. 2017;6(2):187-94. doi:10.1007/s13679-017-0262-y.
114. Zomer E, Gurusamy K, Leach R, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev. 2016;17(10):1001-11. doi:10.1111/obr.12433.
115. Haase CL, Lopes S, Olsen AH, et al. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes (Lond). 2021;45(6):1249-58. doi:10.1038/s41366-021-00788-4.
116. Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023;66(6):965-85. doi:10.1007/s00125-023-05894-8.
117. Rucker D, Padwal R, Li SK, et al. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194-9. doi:10.1136/bmj.39385.413113.25. Erratum in: BMJ. 2007;335(7629). doi:10.1136/bmj.39406.519132.AD.
118. Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet. 2024;403(10434):e21-e31. doi:10.1016/S0140-6736(24)00351-9.
119. Jastreboff AM, Aronne LJ, Ahmad NN, et al.; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387(3):205-16. doi:10.1056/NEJMoa2206038.
120. Kommu S, Sharma PP, Gabor RM. Efficacy and Safety of Tirzepatide on Weight Loss in Patients Without Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes Rev. 2025:e13961. doi:10.1111/obr.13961.
121. Wen J, Syed B, Nadora D, et al. Tirzepatide Versus Semaglutide on Weight Loss in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis of Direct Comparative Studies. Endocrinol Diabetes Metab. 2025;8(3):e70045. doi:10.1002/edm2.70045.
122. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143-55. doi:10.1016/S0140-6736(21)01324-6.
123. Garvey WT, Frias JP, Jastreboff AM, et al.; SURMOUNT-2 investigators. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613-26. doi:10.1016/S0140-6736(23)01200-X.
124. Chuang MH, Chen JY, Wang HY, et al. Clinical Outcomes of Tirzepatide or GLP-1 Receptor Agonists in Individuals With Type 2 Diabetes. JAMA Netw Open. 2024;7(8):e2427258. doi:10.1001/jamanetworkopen.2024.27258.
125. Packer M, Zile MR, Kramer CM, et al.; SUMMIT Trial Study Group. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2025;392(5):427-37. doi:10.1056/NEJMoa2410027.
126. Borlaug BA, Zile MR, Kramer CM, et al. Effects of tirzepatide on circulatory overload and end-organ damage in heart failure with preserved ejection fraction and obesity: a secondary analysis of the SUMMIT trial. Nat Med. 2025;31(2):544-51. doi:10.1038/s41591-024-03374-z.
127. Loomba R, Hartman ML, Lawitz EJ, et al.; SYNERGY-NASH Investigators. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N Engl J Med. 2024;391(4):299-310. doi:10.1056/NEJMoa2401943.
128. Gao X, Hua X, Wang X, et al. Efficacy and safety of semaglutide on weight loss in obese or overweight patients without diabetes: A systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13:935823. doi:10.3389/fphar.2022.935823.
129. Perreault L, Davies M, Frias JP, et al. Changes in Glucose Metabolism and Glycemic Status With Once-Weekly Subcutaneous Semaglutide 2.4 mg Among Participants With Prediabetes in the STEP Program. Diabetes Care. 2022;45(10):2396-405. doi:10.2337/dc21-1785.
130. Zhang R, Hou QC, Li BH, et al. Efficacy and safety of subcutaneous semaglutide in adults with overweight or obese: a subgroup meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2023;14:1132004. doi:10.3389/fendo.2023.11320.
131. de Oliveira Almeida G, Nienkötter TF, Balieiro CCA, et al. Cardiovascular Benefits of GLP-1 Receptor Agonists in Patients Living with Obesity or Overweight: A Meta-analysis of Randomized Controlled Trials. Am J Cardiovasc Drugs. 2024;24(4):509-21. doi:10.1007/s40256-024-00647-3.
132. Davies MJ, Aronne LJ, Caterson ID, et al.; Satiety and Clinical Adiposity — Liraglutide Evidence in individuals with and without diabetes (SCALE) study groups. Liraglutide and cardiovascular outcomes in adults with overweight, or obesity: A post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20(3):734-9. doi:10.1111/dom.13125.
133. Leite AR, Angélico-Gonçalves A, Vasques-Nóvoa F, et al. Effect of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese adults without diabetes: A meta-analysis of placebo-controlled randomized trials. Diabetes Obes Metab. 2022;24(8):1676-80. doi:10.1111/dom.14707.
134. Bandyopadhyay S, Das S, Samajdar SS, Joshi SR. Role of semaglutide in the treatment of nonalcoholic fatty liver disease or non-alcoholic steatohepatitis: A systematic review and meta-analysis. Diabetes Metab Syndr. 2023;17(10):102849. doi:10.1016/j.dsx.2023.102849.
135. Natale P, Green SC, Tunnicliffe DJ, et al. Glucagon-like peptide 1 (GLP-1) receptor agonists for people with chronic kidney disease and diabetes. Cochrane Database Syst Rev. 2025;2(2):CD015849. doi:10.1002/14651858.CD015849.pub2.
136. Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892.
137. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843-54.
138. Davies MJ, Bergenstal R, Bode B, et al. NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687-99.
139. Lin Q, Xue Y, Zou H, et al. Efficacy and safety of liraglutide for obesity and people who are overweight: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol. 2022;15(12):1461-9. doi:10.1080/17512433.2022.2130760.
140. Xie Z, Yang S, Deng W, et al. Efficacy and Safety of Liraglutide and Semaglutide on Weight Loss in People with Obesity or Overweight: A Systematic Review. Clin Epidemiol. 2022;14:1463-76. doi:10.2147/CLEP.S391819.
141. Deng Y, Park A, Zhu L, et al. Effect of semaglutide and liraglutide in individuals with obesity or overweight without diabetes: a systematic review. Ther Adv Chronic Dis. 2022;13:20406223221108064. doi:10.1177/20406223221108064.
142. le Roux CW, Astrup A, Fujioka K, et al.; SCALE Obesity Prediabetes NN8022-1839 Study Group. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399-409. doi:10.1016/S0140-6736(17)30069-7. Erratum in: Lancet. 2017;389(10077):1398. doi:10.1016/S0140-6736(17)30705-5.
143. Song T, Jia Y, Li Z, et al. Effects of Liraglutide on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021;12(6):1735-49. doi:10.1007/s13300-021-01072-4.
144. Kalogirou MS, Patoulias D, Haidich AB, et al. Liraglutide in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Clinics and Research in Hepatology and Gastroenterology. 2021;45(3):101568.
145. Mantovani A, Petracca G, Beatrice G, et al. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites. 2021;11(2):73. doi:10.3390/metabo11020073.
146. Berg S, Stickle H, Rose SJ, Nemec EC. Discontinuing glucagon-like peptide-1 receptor agonists and body habitus: A systematic review and meta-analysis. Obes Rev. 2025:e13929. doi:10.1111/obr.13929.
147. Williamson DF, Pamuk E, Thun M, et al. Prospective Study of Intentional Weight Loss and Mortality in Overweight White Men Aged 40-64 Years. Am J Epidemiol. 1999;149(6):491-03.
148. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study: A randomized study of orlistat as an adjunct to life-style changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155-61.
149. Singh AK, Singh R. Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs. Expert Rev Clin Pharmacol. 2020;13(1):53-64. doi:10.1080/17512433.2020.1698291.
150. Sahebkar A, Simental-Mendía LE, Reiner Ž, et al. Effect of orlistat on plasma lipids and body weight: A systematic review and meta-analysis of 33 randomized controlled trials. Pharmacol Res. 2017;122:53-65. doi:10.1016/j.phrs.2017.05.022.
151. Wilding JP, Jacob S. Cardiovascular outcome trials in obesity: a review. Obesity reviews. 2021;22(1):e13112.
152. James W, Philip T. The SCOUT study: risk-benefit profile of sibutramine in overweight high-risk cardiovascular patients. European heart journal. 2005; supplements 7.suppl_L: L44-L48.
153. Dedov II, Melnichenko GA, Troshina EA, et al. Body Weight Reduction Associated with the Sibutramine Treatment: Overall Results of the PRIMAVERA Primary Health Care Trial. Obes Facts. 2018;11(4):335-43.
154. Дедов И. И., Шестакова М. В., Мельниченко Г. А. и др. Междисциплинарные клинические рекомендации "Лечение ожирения и коморбидных заболеваний". Ожирение и метаболизм. 2021;18(1):5-99. doi:10.14341/omet12714.
155. Bramante CT, Raatz S, Bomberg EM, et al. Cardiovascular Risks and Benefits of Medications Used for Weight Loss. Front Endocrinol (Lausanne). 2020;10:883. doi:10.3389/fendo.2019.00883.
156. Liu L, Li Z, Ye W, et al. Safety and effects of anti-obesity medications on weight loss, cardiometabolic, and psychological outcomes in people living with overweight or obesity: a systematic review and meta-analysis. EClinicalMedicine. 2024;79:103020. doi:10.1016/j.eclinm.2024.103020.
157. James WP, Caterson ID, Coutinho W, et al.; SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905-17. doi:10.1056/NEJMoa1003114.
158. Masson W, Lobo M, Nogueira JP, et al. Anti-inflammatory effect of semaglutide: updated systematic review and meta-analysis. Front Cardiovasc Med. 2024;11:1379189. doi:10.3389/fcvm.2024.1379189.
159. Cho YK, La Lee Y, Jung CH. The Cardiovascular Effect of Tirzepatide: A Glucagon-Like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide Dual Agonist. J Lipid Atheroscler. 2023;12(3):213-22. doi:10.12997/jla.2023.12.3.213.
160. Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications. 2017;31(7):1237-42. doi:10.1016/j.jdiacomp.2016.05.022.
161. Katsiki N, Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: A "promised land" in the COVID-19 era? J Diabetes Complications. 2020;34(12):107723. doi:10.1016/j.jdiacomp.2020.107723.
162. Usman MS, Davies M, Hall ME, et al. The cardiovascular effects of novel weight loss therapies. Eur Heart J. 2023;44(48):5036-48. doi:10.1093/eurheartj/ehad664.
Об авторах
С. В. НедогодаРоссия
Д.м.н., профессор, зав. кафедрой внутренних болезней Института непрерывного медицинского и фармацевтического образования.
Волгоград
Конфликт интересов:
Нет
О. В. Цыганкова
Россия
Д.м.н., доцент, профессор кафедры неотложной терапии с эндокринологией и профпатологией ФПК и ППВ, с.н.с.
Новосибирск
Конфликт интересов:
Нет
Рецензия
Для цитирования:
Недогода С.В., Цыганкова О.В. Избыточная масса тела и ожирение при метаболическом синдроме. Российский кардиологический журнал. 2025;30(1S):6535. https://doi.org/10.15829/1560-4071-2025-6535
For citation:
Nedogoda S.V., Tsygankova O.V. Overweight and obesity in metabolic syndrome. Russian Journal of Cardiology. 2025;30(1S):6535. (In Russ.) https://doi.org/10.15829/1560-4071-2025-6535
JATS XML

















































