Перейти к:
Острый коронарный синдром без подъема сегмента ST электрокардиограммы. Клинические рекомендации 2024
https://doi.org/10.15829/1560-4071-2025-6319
EDN: CXJUIB
Аннотация
Российское кардиологическое общество
При участии: Ассоциации сердечно-сосудистых хирургов России, Российского общества скорой медицинской помощи, Российского научного общества специалистов по рентгенэндоваскулярной диагностике и лечению
Одобрено Научно-практическим Советом Минздрава Российской Федерации.
Ключевые слова
Для цитирования:
Аверков О.B., Арутюнян Г.К., Дупляков Д.В., Константинова Е.В., Никулина Н.Н., Шахнович Р.М., Явелов И.С., Яковлев А.Н., Абугов С.А., Алекян Б.Г., Аронов Д.М., Архипов М.В., Барбараш О.Л., Бойцов С.А., Бубнова М.Г., Вавилова Т.В., Васильева У.Ю., Галявич А.С., Ганюков В.И., Гиляревский С.Р., Голубев Е.П., Голухова Е.З., Затейщиков Д.А., Карпов Ю.А., Космачева Е.Д., Лопатин Ю.М., Марков В.А., Меркулов Е.В., Новикова Н.А., Панченко Е.П., Певзнер Д.В., Погосова Н.В., Прасол Д.М., Протопопов А.В., Скрыпник Д.В., Тарасов Р.С., Терещенко С.Н., Устюгов С.А., Хрипун А.В., Цебровская Е.А., Шалаев С.В., Шляхто Е.В., Шпектор А.В., Якушин С.С. Острый коронарный синдром без подъема сегмента ST электрокардиограммы. Клинические рекомендации 2024. Российский кардиологический журнал. 2025;30(5):6319. https://doi.org/10.15829/1560-4071-2025-6319. EDN: CXJUIB
For citation:
Averkov O.V., Harutyunyan G.K., Duplyakov D.V., Konstantinova E.V., Nikulina N.N., Shakhnovich R.M., Yavelov I.S., Yakovlev A.N., Abugov S.A., Alekyan B.G., Aronov D.M., Arkhipov M.V., Barbarash O.L., Boytsov S.A., Bubnova M.G., Vavilova T.V., Vasilyeva E.Yu., Galyavich A.S., Ganyukov V.I., Gilyarevsky S.R., Golubev E.P., Golukhova E.Z., Zateyshchikov D.A., Karpov Yu.A., Kosmacheva E.D., Lopatin Yu.M., Markov V.A., Merkulov E.V., Novikova N.A., Panchenko E.P., Pevzner D.V., Pogosova N.V., Prasol D.M., Protopopov A.V., Skrypnik D.V., Tarasov R.S., Tereshchenko S.N., Ustyugov S.A., Khripun A.V., Tsebrovskaya E.A., Shalaev S.V., Shlyakhto E.V., Shpektor A.V., Yakushin S.S. 2024 Clinical practice guidelines for Acute coronary syndrome without ST segment elevation electrocardiogram. Russian Journal of Cardiology. 2025;30(5):6319. (In Russ.) https://doi.org/10.15829/1560-4071-2025-6319. EDN: CXJUIB
Список сокращений
- АСБ — атеросклеротическая бляшка
- АВ — атриовентрикулярная
- АВС — активированное время свёртывания крови
- АГ — артериальная гипертензия
- АД — артериальное давление
- АСК — ацетилсалициловая кислота**
- АЧТВ — активированное частичное тромбопластиновое время
- АРА — антагонисты рецепторов ангиотензина II
- БСК — болезни системы кровообращения
- ВАБК — внутриаортальная баллонная контрпульсация
- в/в — внутривенно
- ВИЧ — вирус иммунодефицита человека
- ВСУЗИ — внутрисосудистое ультразвуковое исследование коронарных артерий (ультразвуковое исследование коронарных артерий внутрисосудистое)
- ГМГ-КоА редуктаза — 3-гидрокси-3-метилглутарил коэнзима А редуктаза
- ГП IIb/IIIa — гликопротеины IIb/IIIa
- ДЗЛК — давление заклинивания легочных капилляров
- ЕД (ед.) — единицы действия
- ЕОК — Европейское общество кардиологов
- ЖА — желудочковая аритмия
- ЖТ — желудочковая тахикардия
- иАПФ — ингибиторы ангиотензинпревращающего фермента
- ИБС — ишемическая болезнь сердца
- ИВЛ — искусственная вентиляция лёгких
- Ингибиторы Р2Y12-рецептора тромбоцитов — ингибиторы Р2Y12-рецептора тромбоцитов (АТХ-группа Антиагреганты, кроме гепарина, B01AC)
- ИМ — инфаркт миокарда
- ИМбпST — инфаркт миокарда без стойкого подъема сегмента ST на ЭКГ
- ИМБОКА — инфаркт миокарда без обструктивного поражения коронарных артерий
- ИМпST — инфаркт миокарда со стойким подъемом сегмента ST на ЭКГ
- КА — коронарная артерия
- КГ — коронарография
- КВД — кардиовертер-дефибриллятор (кардиовертер-дефибриллятор имплантируемый однокамерный***; кардиовертер-дефибриллятор имплантируемый двухкамерный***; кардиовертер-дефибриллятор имплантируемый трехкамерный (бивентрикулярный)***)
- КДД — конечное диастолическое давление
- КР — кардиореабилитация
- КТ — компьютерная томография (компьютерная томография органов грудной полости; компьютерная томография органов грудной полости с внутривенным болюсным контрастированием; компьютерная томография грудной полости с внутривенным болюсным контрастированием, мультипланарной и трехмерной реконструкцией)
- КШ — коронарное шунтирование (коронарное шунтирование в условиях искусственного кровообращения; коронарное шунтирование на работающем сердце без использования искусственного кровообращения; коронарное шунтирование роботассистированное; коронарное шунтирование с протезированием клапанов сердца в условиях искусственного кровообращения; коронарное шунтирование с пластикой клапанов сердца в условиях искусственного кровообращения; коронарное шунтирование с протезированием и пластикой клапанов сердца в условиях искусственного кровообращения; коронарное шунтирование в сочетании с трансмиокардиальной лазерной реваскуляризацией сердца; коронарное шунтирование в сочетании с трансмиокардиальной лазерной реваскуляризацией сердца в условиях искусственного кровообращения)
- ЛВП — липопротеиды высокой плотности
- ЛЖ — левый желудочек
- ЛНП — липопротеиды низкой плотности
- ЛНПГ — левая ножка пучка Гиса
- ME — международные единицы
- МЖП — межжелудочковая перегородка
- МНО — международное нормализованное отношение
- МРТ — магнитно-резонансная томография (магнитно-резонансная томография сердца и магистральных сосудов; магнитно-резонансная томография сердца с контрастированием)
- НС — нестабильная стенокардия
- НФГ — нефракционированный гепарин (син.: гепарин натрия**)
- ОКС — острый коронарный синдром
- ОКСбпST — острый коронарный синдром без стойкого подъема сегмента ST на ЭКГ
- ОКСпST — острый коронарный синдром со стойким подъемом сегмента ST на ЭКГ
- ОКТ — оптическая когерентная томография коронарных артерий
- ОСН — острая сердечная недостаточность
- ПЖ — правый желудочек
- рСКФ — расчётная скорость клубочковой фильтрации
- РФ — Российская Федерация
- СБЛП — стент без лекарственного покрытия (син.: стент для коронарных артерий металлический непокрытый***)
- СВЛ — стент, выделяющий лекарственное средство (стент для коронарных артерий, выделяющий лекарственное средство, полностью рассасывающийся***; стент для коронарных артерий, выделяющий лекарственное средство, с рассасывающимся полимерным покрытием***; стент для коронарных артерий, выделяющий лекарственное средство, с нерассасывающимся полимерным покрытием***)
- СД — сахарный диабет
- СИ — сердечный индекс
- СН — сердечная недостаточность
- ТП — трепетание предсердий
- ТЭЛА — тромбоэмболия легочных артерий
- УДД — уровень достоверности доказательств
- УЗИ — ультразвуковое исследование
- УУР — уровень убедительности рекомендаций
- ФВ — фракция выброса
- ФЖ — фибрилляция желудочков
- ФК — функциональный класс
- ФРК — фракционный резерв коронарного кровотока
- ФП — фибрилляция предсердий
- ХБП — хроническая болезнь почек
- ХС — холестерин
- ХСН — хроническая сердечная недостаточность
- ЦВД — центральное венозное давление
- ЧКВ — чрескожное коронарное вмешательство (транслюминальная баллонная ангиопластика коронарных артерий; стентирование коронарной артерии; транслюминальная баллонная ангиопластика и стентирование коронарных артерий; реканализация коронарных артерий ретроградная со стентированием; реканализация коронарных артерий антеградная со стентированием; тромбоэктомия из сосудистого протеза (коронарного); эмболэктомия (из коронарной артерии); попытка стентирования коронарных артерий)
- ЧСС — частота сердечных сокращений
- ЭКГ — электрокардиография (син.: регистрация электрокардиограммы), электрокардиограмма
- ЭКМО — экстракорпоральная мембранная оксигенация
- ЭКС — электрокардиостимулятор (электрокардиостимулятор имплантируемый двухкамерный, без частотной адаптации***; электрокардиостимулятор имплантируемый двухкамерный, частотно-адаптивный***; электрокардиостимулятор имплантируемый однокамерный, без частотной адаптации***; электрокардиостимулятор имплантируемый однокамерный, частотно-адаптивный***; электрокардиостимулятор имплантируемый трехкамерный (бивентрикулярный)***; электрокардиостимулятор имплантируемый двухкамерный, частотно-адаптивный, совместимый с магнитно-резонансным томографом***)
- ЭхоКГ — эхокардиография
- ARC-HBR — Academic Research Consortium for High Bleeding Risk (шкала высокого риска кровотечений Консорциумa академических исследований)
- β-АБ — бета-адреноблокаторы
- NYHA — New York Heart Association (Нью-Йоркская ассоциация сердца)
- PaO2 — парциальное давление кислорода в артериальной крови
- PCSK9 — proprotein convertase subtilisin/kexin type 9 (пропротеиновая конвертаза субтилизин-кексинового типа 9)
- POC — point of care (место оказания медицинской помощи)
- SpO2 — уровень насыщения крови кислородом (сатурация крови), определяемый пульсоксиметром
Особые обозначения лекарственных препаратов и медицинских изделий
Дополнительными указательными значками обозначены: ** — лекарственные средства в случае, если тезис-рекомендация относится к лекарственному препарату для медицинского применения, внесенному в перечень жизненно необходимых и важнейших лекарственных препаратов для медицинского применения, *** — медицинские изделия в случае, если тезис-рекомендация относится к медицинскому изделию, имплантируемому в организм человека при оказании медицинской помощи в рамках программы государственных гарантий бесплатного оказания гражданам медицинской помощи, # — лекарственные средства в случае, если тезис-рекомендация относится к лекарственному препарату для медицинского применения, используемому в несоответствии с показаниями к применению и противопоказаниями и/или способами применения и дозами, содержащимися в инструкции по применению лекарственного препарата (off label).
Термины и определения
Доказательная медицина — надлежащее, последовательное и осмысленное использование современных наилучших доказательств (результатов клинических исследований) в процессе принятия решений о состоянии здоровья и лечении пациента [1][2].
Заболевание — возникающее в связи с воздействием патогенных факторов нарушение деятельности организма, работоспособности, способности адаптироваться к изменяющимся условиям внешней и внутренней среды при одновременном изменении защитно-компенсаторных и защитно-приспособительных реакций и механизмов организма1.
Избирательная инвазивная стратегия лечения острого коронарного синдрома без стойкого подъема сегмента ST (ОКСбпST) на электрокардиограмме (ЭКГ) — диагностическая коронарография (КГ) для решения вопроса о целесообразности немедленной реваскуляризации миокарда только при появлении или возобновлении ишемии миокарда (в т. ч. в ходе неинвазивных стресс-тестов) или возникновении серьезных осложнений (острая сердечная недостаточность (ОСН), злокачественные желудочковые аритмии (ЖА)) [3].
Инструментальная диагностика — диагностика с использованием различных приборов, аппаратов и инструментов.
Инфаркт миокарда без стойкого подъема сегмента ST на ЭКГ (ИМбпST) — инфаркт миокарда (ИМ), при котором в ранние сроки заболевания на ЭКГ отсутствует стойкий (длительностью >20 мин) подъем сегмента ST как минимум в двух смежных отведениях и нет остро возникшей блокады левой ножки пучка Гиса (ЛНПГ).
Исход — любой возможный результат, возникающий от воздействия причинного фактора, профилактического или терапевтического вмешательства, все установленные изменения состояния здоровья, возникающие как следствие вмешательства2.
Конфликт интересов — ситуация, при которой у медицинского или фармацевтического работника при осуществлении ими профессиональной деятельности возникает личная заинтересованность в получении лично либо через представителя компании материальной выгоды или иного преимущества, которое влияет или может повлиять на надлежащее исполнение ими профессиональных обязанностей вследствие противоречия между личной заинтересованностью медицинского работника или фармацевтического работника и интересами пациента1.
Клиническое исследование — любое исследование, проводимое с участием человека в качестве субъекта для выявления или подтверждения клинических и/или фармакологических эффектов исследуемых продуктов, и/или выявления нежелательных реакций на исследуемые продукты, и/или изучения их всасывания, распределения, метаболизма и выведения с целью оценить их безопасность и/или эффективность. Термины "клиническое испытание" и "клиническое исследование" являются синонимами3.
Лабораторная диагностика — совокупность методов, направленных на анализ исследуемого материала с помощью различного специализированного оборудования.
Лекарственные препараты — лекарственные средства в виде лекарственных форм, применяемые для профилактики, диагностики, лечения заболевания, реабилитации, для сохранения, предотвращения или прерывания беременности4.
Медицинское вмешательство — выполняемые медицинским работником и иным работником, имеющим право на осуществление медицинской деятельности, по отношению к пациенту, затрагивающие физическое или психическое состояние человека и имеющие профилактическую, диагностическую, лечебную, реабилитационную или исследовательскую направленность виды медицинских обследований и/или медицинских манипуляций, а также искусственное прерывание беременности1.
Медицинский работник — физическое лицо, которое имеет медицинское или иное образование, работает в медицинской организации и в трудовые (должностные) обязанности которого входит осуществление медицинской деятельности, либо физическое лицо, которое является индивидуальным предпринимателем, непосредственно осуществляющим медицинскую деятельность1.
Механический протез клапана сердца — протез аортального клапана механический двустворчатый***; протез митрального клапана механический двустворчатый***; протез аортального клапана механический двустворчатый в сочетании с протезом аорты из биологического полимера***.
Мониторирование ЭКГ — дистанционное наблюдение за электрокардиографическими данными.
Неинвазивные стресс-тесты — ЭКГ с физической нагрузкой, ЭКГ с применением лекарственных препаратов, эхокардиография (ЭхоКГ) с физической нагрузкой, ЭхоКГ с фармакологической нагрузкой, сцинтиграфия миокарда с функциональными пробами, однофотонная эмиссионная компьютерная томография (КТ) миокарда перфузионная с функциональными пробами.
Неотложная инвазивная стратегия лечения ОКСбпST на ЭКГ — диагностическая КГ в первые 2 ч после госпитализации для решения вопроса о целесообразности немедленной реваскуляризации миокарда [3].
Нестабильная стенокардия (НС) — недавно возникшая или утяжелившаяся стенокардия, когда тяжесть и продолжительность ишемии недостаточны для развития острого ишемического повреждения миокарда (который диагностируется по превышению 99-го перцентиля верхней референсной границы у пациентов без исходного повышения уровня сердечного тропонина в крови, либо повышению и/или снижению концентрации сердечного тропонина >20% при его исходно стабильно (вариабельность была ≤20%) повышенном уровне). В понятие "нестабильная стенокардия" входят: длительный (>20 мин) ангинозный приступ в покое; или впервые возникшая стенокардия, соответствующая как минимум II функциональному классу (ФК) по классификации Канадского сердечно-сосудистого общества; или утяжелившаяся стенокардия (прогрессирующая стенокардия, стенокардия crescendo) как минимум до III ФК по классификации Канадского сердечно-сосудистого общества; или стенокардия, появившаяся в первые 2 нед. после ИМ (постинфарктная стенокардия).
Операция коронарного шунтирования (КШ) — наложение обходного анастомоза, позволяющего улучшить кровоток дистальнее гемодинамически значимого стеноза коронарной артерии (КА). В зависимости от методики включает аортокоронарное, маммарокоронарное и другие виды шунтирования.
Острое повреждение миокарда — повышение и/или снижение концентрации сердечного тропонина в крови, которая как минимум однократно превышает 99-й перцентиль верхней референсной границы у пациентов без исходного повышения уровня сердечного тропонина в крови, либо его увеличение >20% при исходно повышенном уровне сердечного тропонина, если до этого он оставался стабильным (вариабельность была ≤20%) или снижался.
Острый ИМ — острое повреждение миокарда вследствие его ишемии.
Острый коронарный синдром (ОКС) — термин, обозначающий любую группу клинических признаков или симптомов, позволяющих подозревать острый ИМ или НС.
ОКС со стойким подъемом сегмента ST на ЭКГ (ОКСпST) — остро возникшие клинические признаки или симптомы ишемии миокарда в сочетании с наличием стойкого (длительностью >20 мин) подъема сегмента ST как минимум в двух смежных отведениях ЭКГ.
ОКСбпST — остро возникшие клинические признаки или симптомы ишемии миокарда, когда на ЭКГ отсутствует стойкий (длительностью >20 мин) подъем сегмента ST как минимум в двух смежных отведениях и нет остро возникшей блокады ЛНПГ.
Отсроченная инвазивная стратегия лечения ОКСбпST на ЭКГ — диагностическая КГ для решения вопроса о целесообразности немедленной реваскуляризации миокарда до 72 ч после госпитализации.
Пациент — физическое лицо, которому оказывается медицинская помощь или которое обратилось за оказанием медицинской помощи независимо от наличия у него заболевания и от его состояния1.
Постинфарктная стенокардия — стенокардия, возникшая в первые 2 нед. от начала ИМ.
Рабочая группа по разработке/актуализации клинических рекомендаций — коллектив специалистов, работающих совместно и согласованно в целях разработки/актуализации клинических рекомендаций, и несущих общую ответственность за результаты данной работы.
Ранняя инвазивная стратегия лечения ОКСбпST на ЭКГ — диагностическая КГ для решения вопроса о целесообразности немедленной реваскуляризации миокарда в первые 24 ч после госпитализации [3].
Реваскуляризация — выполнение процедур, входящих в понятия чрескожного коронарного вмешательства (ЧКВ) и/или КШ, в любых сочетаниях.
Ресинхронизирующая терапия — имплантация пациенту специального бивентрикулярного (трехкамерного) электрокардиостимулятора (ЭКС) для ресинхронизации сердечных сокращений (кардиовертера-дефибриллятора имплантируемого трехкамерного (бивентрикулярного)*** (CRT-D); ЭКС имплантируемого трехкамерного (бивентрикулярного)***, электростимулятора имплантируемого трехкамерного (бивентрикулярного)***, магнитно-резонансная томография (МРТ) совместимого (CRT-P)).
Симптом — признак какого-либо заболевания, статистически значимое отклонение того или иного показателя от границ его нормальных величин или возникновение качественно нового, не свойственного здоровому организму явления.
Синдром — устойчивая совокупность ряда симптомов с единым патогенезом5.
Состояние — изменения организма, возникающие в связи с воздействием патогенных и/или физиологических факторов и требующие оказания медицинской помощи1.
Стент без лекарственного покрытия (СБЛП) — стент для КА (коронарный стент) без лекарственного покрытия (голометаллический, металлический непокрытый); представляет собой металлический каркас из биологически инертного материала.
Стент, выделяющий лекарство (СВЛ) — стент для КА, из структур которого в течение определенного времени после установки выделяется антипролиферативное вещество, препятствующее образованию неоинтимы и за счет этого способствующее профилактике повторного стенозирования.
Тезис-рекомендация — положение, отражающее порядок и правильность выполнения того или иного медицинского вмешательства, имеющего доказанную эффективность и безопасность.
Уровень достоверности доказательств (УДД) — степень уверенности в том, что найденный эффект от применения медицинского вмешательства является истинным [4].
Уровень убедительности рекомендаций (УУР) — степень уверенности в достоверности эффекта вмешательства и в том, что следование рекомендациям принесет больше пользы, чем вреда в конкретной ситуации [4].
Хирургическое лечение — метод лечения заболеваний путём разъединения и соединения тканей в ходе хирургической операции.
Чрескожное коронарное вмешательство (ЧКВ) — восстановление кровотока в стенозированном участке КА с использованием чрескожного введения необходимых для этого устройств.
Этапная реваскуляризация — последовательное (т. е. не одновременное с реваскуляризацией проблемного сегмента КА, ответственного за данный ОКСбпST) выполнение процедур, входящих в понятия ЧКВ и/или КШ, в любых сочетаниях, во время индексной госпитализации или после нее с целью полной или максимально возможной реваскуляризации коронарного русла.
CHA2DS2-VASc — шкала риска ишемического инсульта при фибрилляции предсердий (ФП) у пациентов без механического протеза клапана сердца и умеренного или тяжелого митрального стеноза.
1. Краткая информация по заболеванию или состоянию (группе заболеваний или состояний)
1.1. Определение заболевания или состояния (группы заболеваний или состояний)
ОКС — термин, обозначающий любую группу клинических признаков или симптомов, позволяющих подозревать ИМ или НС. Термин "ОКС" используется, когда недостаточно диагностических критериев для нозологического диагноза ("ИМ" или "НС") и, следовательно, представляет собой предварительный диагноз в первые часы и сутки заболевания, в то время как термины "ИМ" и "НС" используются при формулировании окончательного диагноза. Соответственно, термин "ОКС" может использоваться на догоспитальном или раннем госпитальном этапах и в дальнейшем трансформируется в диагноз "острый ИМ", "НС" либо, по результатам дифференциальной диагностики, — в любой другой диагноз, в т. ч. некардиологический [3].
ОКС может быть как проявлением дестабилизации хронического течения ишемической болезни сердца (ИБС), так и первым проявлением поражения коронарного русла у пациентов, не предъявлявших ранее каких-либо кардиологических жалоб.
ОКСбпST на ЭКГ — остро возникшие клинические признаки или симптомы ишемии миокарда при отсутствии на ЭКГ стойкого (длительностью >20 мин) подъема сегмента ST как минимум в двух смежных отведениях и при отсутствии остро возникшей блокады ЛНПГ.
ИМ — острое повреждение миокарда вследствие его ишемии [5]. Для диагностики острого ИМ, не связанного с ЧКВ или операцией КШ, следует документировать повышение и/или снижение концентрации сердечного тропонина в крови, которая должна как минимум однократно превысить 99-й перцентиль верхней референсной границы у пациентов без исходного повышения уровня сердечного тропонина в крови, либо увеличение и/или снижение концентрации сердечного тропонина >20% при его исходно стабильно (вариабельность была ≤20%) повышенном уровне, в сочетании с хотя бы одним критерием острой ишемии миокарда.
Критерии ишемии миокарда:
- остро возникшие (или предположительно остро возникшие) ишемические изменения на ЭКГ;
- появление патологических зубцов Q на ЭКГ;
- подтверждение с помощью методов визуализации наличия новых участков миокарда с потерей жизнеспособности или нарушением локальной сократимости, характерных для ишемической этиологии;
- выявление внутрикоронарного тромбоза при КГ или на аутопсии.
Подробнее критерии ИМ представлены в Приложении А3. Критерии диагностики ИМ [5].
Изменения на ЭКГ, характерные для ишемии миокарда:
- Остро возникшие подъемы сегмента ST на уровне точки J как минимум в двух смежных отведениях ЭКГ ≥0,1 мВ во всех отведениях, за исключением отведений V2-V3, где элевация сегмента ST должна составлять ≥0,2 мВ у мужчин в возрасте 40 лет и старше, ≥0,25 мВ у мужчин моложе 40 лет или ≥0,15 мВ у женщин (при отсутствии гипертрофии левого желудочка (ЛЖ) или блокады ЛНПГ).
- Остро возникшие подъемы сегмента ST на уровне точки J ≥0,1 мВ в отведениях V2-V3 в сравнении с ранее зарегистрированной ЭКГ (при отсутствии гипертрофии ЛЖ или блокады ЛНПГ).
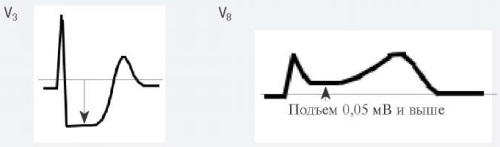
- Остро возникшие горизонтальные или косонисходящие снижения сегмента ST ≥0,05 как минимум в двух смежных отведениях ЭКГ и/или инверсии зубца Т >0,1 мВ как минимум в двух смежных отведениях ЭКГ с доминирующим зубцом R или соотношением амплитуды зубцов R/S >1 [5].
ИМбпST — ИМ, при котором в ранние сроки заболевания на ЭКГ отсутствует стойкий (длительностью >20 мин) подъем сегмента ST как минимум в двух смежных отведениях и отсутствует остро возникшая блокада ЛНПГ.
НС — недавно возникшая или утяжелившаяся стенокардия, когда тяжесть и продолжительность ишемии недостаточны для развития острого ишемического повреждения миокарда (который диагностируется по превышению 99-го перцентиля верхней референсной границы у пациентов без исходного повышения уровня сердечного тропонина в крови, либо повышению и/или снижению концентрации сердечного тропонина >20% при его исходно стабильно (вариабельность была ≤20%) повышенном уровне).
В понятие "нестабильная стенокардия" входят: длительный (>20 мин) ангинозный приступ в покое; или впервые возникшая стенокардия, соответствующая как минимум II ФК по классификации Канадского сердечно-сосудистого общества; или утяжелившаяся стенокардия (прогрессирующая стенокардия, стенокардия crescendo) как минимум до III ФК по классификации Канадского сердечно-сосудистого общества; или стенокардия, появившаяся в первые 2 нед. после ИМ (постинфарктная стенокардия).
В данных рекомендациях рассматриваются диагностические и лечебные подходы не только в тот период, когда осуществляется дифференциальная диагностика ОКСбпST и ОКСпST на ЭКГ или дифференциальная диагностика внутри ОКСбпST (ИМбпST и НС), но и после установления нозологических диагнозов (ИМбпST или НС). В связи с этим приведенные в данном документе рекомендации для диагноза "ОКСбпST" справедливы и для диагнозов "ИМбпST" и "НС".
1.2. Этиология и патогенез заболевания или состояния (группы заболеваний или состояний)
ОКС, как правило, является следствием тромбоза КА. Тромб возникает чаще всего на месте разрыва так называемой ранимой (нестабильной) атеросклеротической бляшки (АСБ) — с большим липидным ядром, богатой воспалительными элементами и истонченной покрышкой, — однако возможно образование тромба и на дефекте эндотелия (эрозии) КА над АСБ. Во многих случаях острый тромбоз возникает в месте исходно гемодинамически незначимого стеноза КА.
В отличие от ОКСпST на ЭКГ при ОКСбпST отсутствует длительная окклюзия крупной КА, вызывающая трансмуральную ишемию миокарда.
В КА пациентов с ОКСбпST нередко находят несколько "уязвимых" АСБ, в т. ч. с нарушенной целостностью их поверхностных структур. Из-за высокого риска возникновения повторных окклюзий КА требуется сочетать у таких пациентов локальные воздействия (на уровне конкретной АСБ, обусловившей развитие клинической картины ОКСбпST) с системными лечебными мероприятиями, направленными на снижение вероятности повреждения других АСБ и формирования атеротромбоза.
Тромб, расположенный на поверхности и даже в структурах АСБ, может быть источником эмболий в дистальное сосудистое русло сердца. Эмболизация микрососудов миокарда сама по себе может приводить к образованию мелких очагов некроза и/или способствовать расширению основного участка некроза. Кроме того, в случае устранения окклюзии КА, мелкие тромбоэмболы могут нарушать кровоток на уровне микроциркуляции, препятствуя полноценному восстановлению кровоснабжения миокарда (реперфузии).
ОКСбпST может быть также следствием спазма КА, спонтанной диссекции (расслоения) КА, а также эмболизации КА, не связанной с атеросклерозом. Ишемию миокарда могут спровоцировать или утяжелить анемия, гипоксемия, воспаление, инфекция, лихорадка, а также метаболические или эндокринные расстройства (в частности, гипертиреоз). Спазм, диссекция и тромбоз КА наряду с тахикардией и повышением артериального давления (АД) могут возникнуть при применении кокаина и некоторых других запрещенных веществ.
У части пациентов с ОКСбпST развивается ишемическое повреждение и в дальнейшем некроз миокарда (ИМ), размеры которого могут быть различными. Следствием потери существенной части активного миокарда является процесс ремоделирования сердца. Образование очага некроза в миокарде сопровождается изменением размера, формы и толщины стенки ЛЖ, а сохранившийся миокард испытывает повышенную нагрузку и подвергается гипертрофии. Насосная функция изменившего форму ЛЖ ухудшается, что ведет к развитию сердечной недостаточности (СН). Наличие даже небольших очагов ишемического некроза миокарда свидетельствует о высоком риске неблагоприятного течения заболевания.
1.3. Эпидемиология заболевания или состояния (группы заболеваний или состояний)
Болезни системы кровообращения (БСК) являются ведущей причиной смертей у взрослого населения Российской Федерации (РФ). В последние годы доля БСК в структуре причин смертности составляет >40%: 2019г — 46%, 2020г — 45%, 2021г — 38% (снижение за счет большой доли новой коронавирусной инфекции (COVID-19) — 17%), 2022г — 44% от общего числа смертельных исходов. В структуре смертности от БСК на долю ИБС в 2022г пришлось 42,3%. Примерно такой же вклад ИБС в смертность от БСК фиксируется в большинстве стран и регионов [6]. В этом же году ИМ как причина смерти зарегистрирован у 48911 человек (5,9% в структуре смертности от БСК)6.
По данным мониторинга Минздрава России, в 2022г в РФ зарегистрировано 438315 случаев ОКС (на 12,5% меньше, чем в 2021г), в т. ч. 150845 случаев ОКСпST и 287470 — ОКСбпST. Диагноз острого ИМ (включая повторный) поставлен 219240 пациентам, из них во время госпитализации умерло 23797, т. е. 10,9%. Наблюдается положительная динамика в снижении госпитальной летальности — этот же показатель в 2018г составил 17,7%. По данным национальных регистров в Европе летальность в стационаре находится в интервале от 4 до 12%. Госпитальная летальность при ОКС в целом в РФ в 2022г составила 5,6%, при ОКСбпST — 2,9%. Очевидно, что летальность при ИМбпST намного выше, чем при НС, но отдельный анализ не проводился. В последние годы соотношение между ИМ со стойким подъемом сегмента ST (ИМпST) и ИМбпST неуклонно меняется в сторону увеличения доли ИМбпST [7], в основном за счет лабораторного выявления "дополнительных" случаев ИМбпST среди тех, которые еще недавно относились к НС. Объективной предпосылкой для этого стало широкое использование для подтверждения острого повреждения миокарда сердечных тропонинов, в т. ч. определяемых высокочувствительными методами.
Частота проведения ЧКВ больным с ОКСбпST остается невысокой — 37%. Следует помнить, что риск осложнений после ОКСбпST, особенно после ИМбпST, при длительном наблюдении такой же высокий как при ИМпST. Это подтверждается результатами регистра Рекорд-3 и зарубежными данными [8][9]. Следует обратить особое внимание на возможности инвазивного лечения пациентов с ОКСбпST.
Несмотря на очевидный прогресс в лечении ОКС, среднесрочный и долгосрочный прогноз остается в целом неблагоприятный. По данным наблюдательных исследований, частота значимых сердечно-сосудистых осложнений (сердечная смерть, повторный ИМ, ишемический инсульт) после ИМ составляет ~20%. В последующие 3 года значимые сердечно-сосудистые осложнения наблюдаются еще у 20% пациентов [10][11]. Следовательно, пациенты после ИМ/ОКС относятся к категории очень высокого риска осложнений, что диктует необходимость диспансерного наблюдения и проведения эффективной вторичной профилактики, как медикаментозной, так и немедикаментозной.
1.4. Особенности кодирования заболевания или состояния (группы заболеваний или состояний) по Международной статистической классификации болезней и проблем, связанных со здоровьем
I20.0 Нестабильная стенокардия.
I21. Острый инфаркт миокарда.
I21.0. Острый трансмуральный инфаркт передней стенки миокарда.
I21.1. Острый трансмуральный инфаркт нижней стенки миокарда.
I21.2. Острый трансмуральный инфаркт миокарда других уточненных локализаций.
I21.3. Острый трансмуральный инфаркт миокарда неуточненной локализации.
I21.4. Острый субэндокардиальный инфаркт миокарда.
I21.9. Острый инфаркт миокарда неуточненный.
I22. Повторный инфаркт миокарда.
I22.0. Повторный инфаркт передней стенки миокарда.
I22.1. Повторный инфаркт нижней стенки миокарда.
I22.8. Повторный инфаркт миокарда другой уточненной локализации.
I22.9. Повторный инфаркт миокарда неуточненной локализации.
I24. Другие формы острой ишемической болезни сердца.
I24.8. Другие формы острой ишемической болезни сердца.
I24.9. Острая ишемическая болезнь сердца неуточненная.
Чаще всего исходом предварительного диагноза "ОКCбпST" является заключительный диагноз:
— "ИМ без формирования патологических зубцов Q" (ему соответствуют рубрики I21.4, I22.0-I22.8);
— "НС" (рубрика I20.0).
В случае, если ОКСбпST прогрессирует в ИМ с формированием зубца Q на ЭКГ, присваиваются коды I21.0-I21.2, I22.0-I22.8.
В редких случаях смерти пациента с ОКСбпST (см. критерии ИМ 3 типа по Четвертому универсальному определению) следует использовать рубрику I24.8.
Так называемые "неуточненные" рубрики (I21.3, I21.9, I22.9, I24.9) и соответствующие им формулировки в заключительном клиническом диагнозе могут использоваться только в исключительных случаях — при наличии объективных трудностей диагностики. В патологоанатомических и судебно-медицинских диагнозах использоваться не могут.
Использование кодов Раздела I23 "Некоторые текущие осложнения острого ИМ" при кодировании основного заболевания/первоначальной причины смерти недопустимо (данные состояния являются осложнением основного заболевания — ИМ).
После 28 сут. от начала симптомов ИМбпST диагноз "ИМ" не применяется. В таком случае принято указывать на перенесенный ранее ИМ, обозначая это состояние как "Постинфарктный кардиосклероз".
1.5. Классификация заболевания или состояния (группы заболеваний или состояний)
Классификации ОКС и острого ИМ:
На этапе предварительного диагноза:
- ОКСпST — ИМ с подъемом сегмента ST (в настоящем документе не рассматривается);
- ОКСбпST.
Клинический диагноз (в т. ч. заключительный) после подтверждения/исключения ИМ:
- ИМ с подъемом сегмента ST (в настоящем документе не рассматривается);
- ИМбпST;
- НС.
Классификация ИМ на основании последующих изменений на ЭКГ (не обязательна к применению):
- ИМ с формированием патологических зубцов Q;
- ИМ без формирования патологических зубцов Q.
Классификация ИМ на основании глубины поражения мышечного слоя (является приоритетной для патологоанатомического/судебно-медицинского диагноза):
- Субэндокардиальный ИМ;
- Трансмуральный ИМ.
Классификация ИМ на основании локализации очага острого ишемического повреждения/некроза:
- ИМ передней стенки ЛЖ (передний ИМ);
- ИМ боковой стенки ЛЖ (боковой ИМ);
- ИМ верхушки сердца;
- ИМ нижней стенки ЛЖ (нижний ИМ);
- ИМ задней стенки ЛЖ (задний ИМ);
- ИМ межжелудочковой перегородки (МЖП);
- ИМ правого желудочка (ПЖ);
- ИМ предсердий;
- Возможны сочетанные локализации: задненижний, переднебоковой и др.
Классификация ИМ на основании наличия ИМ в анамнезе:
- Повторный ИМ — ИМ, развившийся после 28 сут. от начала предшествующего ИМ;
- Рецидив ИМ — ИМ, развившийся в течение 28 сут. (включительно) от начала предшествующего ИМ.
Классификация типов ИМ:
Тип 1. ИМ, развившийся вследствие разрыва или эрозии АСБ в КА с последующим формированием внутрикоронарного тромба (атеротромбоз) с резким снижением кровотока дистальнее поврежденной АСБ или дистальной эмболизацией тромботическими массами/фрагментами АСБ с последующим развитием некроза миокарда. Более редкой причиной ИМ 1 типа является интрамуральная гематома в поврежденной АСБ с быстрым увеличением ее объема и уменьшением просвета артерии.
Тип 2. ИМ, развившийся в результате ишемии, вызванной причинами, не связанными с тромботическими осложнениями коронарного атеросклероза. Патофизиологически такие ИМ связаны с повышением потребности миокарда в кислороде и/или уменьшением его доставки к миокарду, например, вследствие эмболии КА, спонтанной диссекции КА, дыхательной недостаточности, анемии, нарушений ритма сердца, артериальной гипертензии (АГ) или гипотензии и т. д. ИМ 2 типа может возникать как у пациентов с наличием, так и у пациентов с отсутствием коронарного атеросклероза.
Тип 3. ИМ 3 типа соответствует случаям появления симптомов, указывающих на ишемию миокарда, сопровождающихся предположительно новыми ишемическими изменениями ЭКГ или фибрилляцией желудочков (ФЖ), когда пациенты умирают до появления возможности взятия образцов крови или в период до повышения активности биохимических маркеров некроза миокарда в крови. Диагноз подтверждается на основании обнаружения острого ИМ на аутопсии.
Тип 4a. ИМ, связанный с осложнениями, возникшими по время процедуры ЧКВ и в ближайшие 48 ч после нее.
Тип 4b. ИМ, связанный с тромбозом коронарного стента, документированный при КГ или аутопсии. В зависимости от сроков после имплантации стента выделяют острый (0-24 ч), подострый (>24 ч — 30 сут.), поздний (>30 сут. — 1 год) и очень поздний (>1 года) тромбоз стента.
Тип 4c. ИМ, связанный с рестенозом после ЧКВ. ИМ 4с типа устанавливается в случае обнаружения выраженного рестеноза в артерии, соответствующей зоне ИМ, когда отсутствуют признаки тромбоза и другие поражения инфаркт-связанной артерии.
Тип 5. ИМ, связанный с операцией КШ.
Критерии диагностики и дифференциальной диагностики ИМ разных типов представлены в Приложении А3. Критерии диагностики ИМ [5].
Клинические варианты НС:
- Стенокардия покоя при болевом синдроме >20 мин;
- Впервые возникшая стенокардия, соответствующая как минимум II ФК по классификации Канадского сердечно-сосудистого общества;
- Утяжелившаяся стенокардия (прогрессирующая стенокардия, син.: стенокардия crescendo) как минимум до III ФК по классификации Канадского сердечно-сосудистого общества;
- Постинфарктная стенокардия (появившаяся в первые 2 нед. после ИМ).
В связи с тем, что не каждый случай стенокардии покоя, впервые возникшей стенокардии или утяжелившейся стенокардии соответствует критериям НС, в случаях ОКСбпST при исключении ИМ диагноз оптимально формулировать как "Нестабильная стенокардия" (код I20.0).
1.6. Клиническая картина заболевания или состояния (группы заболеваний или состояний)
Для ишемии/повреждения/некроза миокарда характерны боль или чувство сжатия, давления или тяжести за грудиной, которые иногда описываются пациентом как дискомфорт. Возможны иррадиация в левую руку, левое плечо, горло, нижнюю челюсть, эпигастрий, а также нетипичные клинические проявления, такие как потливость, тошнота, боль в животе, одышка, удушье, потеря сознания, которые в некоторых случаях являются единственными или доминирующими.
При ОКС симптомы, как правило, сходны по характеру с возникающими при приступе стенокардии, но отличаются по силе и продолжительности; в ряде случаев симптомы полностью не купируются приемом нитроглицерина**, а иногда — и повторными инъекциями наркотических анальгетиков. Интенсивность болевого синдрома может быть различной — от незначительной до невыносимой; симптомы могут носить волнообразный характер и продолжаться от 20 мин до нескольких часов.
ОКСбпST следует заподозрить в следующих клинических ситуациях: (1) длительный (>20 мин) ангинозный приступ в покое; (2) впервые возникшая стенокардия, соответствующая как минимум II ФК по классификации Канадского сердечно-сосудистого общества; (3) утяжеление до этого стабильной стенокардии как минимум до III ФК по классификации Канадского сердечно-сосудистого общества (стенокардия crescendo); (4) стенокардия, появившаяся в первые 2 нед. после ИМ (постинфарктная стенокардия).
При нетипичных клинических проявлениях в зависимости от доминирующей симптоматики у пациентов с развивающимся ИМ выделяют астматический вариант, абдоминальный вариант, аритмический вариант, цереброваскулярный вариант, а также малосимптомную (безболевую) форму. Аналогичные проявления возможны и при острой ишемии миокарда без формирования ИМ. В клинической картине ОКСбпST могут присутствовать, иногда преобладать, симптомы его основных осложнений — ОСН (отек легких, кардиогенный шок), выраженной брадикардии или тахикардии и др.
2. Диагностика заболевания или состояния (группы заболеваний или состояний), медицинские показания и противопоказания к применению методов диагностики
Критерии установления диагноза ОКСбпST представлены в Разделе 1.5. Классификация заболевания или состояния (группы заболеваний или состояний): клинические варианты нестабильной стенокардии и Приложении А3. Критерии диагностики ИМ.
2.1. Жалобы и анамнез
В начальной диагностике ОКСбпST рекомендуется опираться на оценку характера болевого синдрома и/или его эквиваленты. Достаточно типична иррадиация боли в шею, нижнюю челюсть и левую руку. Однако у ряда пациентов на первый план могут выходить менее характерные симптомы — одышка, боль в эпигастрии, тошнота, головокружение, слабость и другие. Положительный эффект на нитроглицерин не исключает ОКСбпST.
При первичном медицинском контакте с пациентом рекомендуется опираться на клинические проявления заболевания, изменения на ЭКГ, а также фиксировать время от начала болевого эпизода до контакта с врачом (медицинским работником). В отдельных случаях дифференциальной диагностики с другими состояниями или заболеваниями, сопровождающимися изменениями на ЭКГ, следует сосредоточиться на подробном изучении характеристик болевого синдрома, а также данных других, в первую очередь визуализирующих (например, ЭхоКГ, мультиспиральной КТ) и лабораторных методов.
- У всех пациентов с подозрением на ОКСбпST рекомендуется сбор и оценка жалоб и анамнеза для определения особенностей обострения ИБС, проведения дифференциальной диагностики, выявления симптомов, позволяющих оценить наличие и тяжесть других заболеваний, провоцирующих или осложняющих течение ОКСбпST и способных повлиять на выбор подходов к лечению, а также выявить осложнения ОКСбпST [3].
ЕОК IС (УУР С, УДД 5)
Комментарии. Клинические проявления ОКСбпST многообразны — от остановки сердечной деятельности, шока, угрожающих жизни аритмий до минимальных жалоб в виде небольшого дискомфорта за грудиной/одышки, а в ряде случаев и отсутствии какой-либо клинической симптоматики. До 50% пациентов, направленных в стационар с ОКСбпST, будут выписаны с другим диагнозом, в т. ч. не имеющим отношения к заболеваниям сердечно-сосудистой системы.
Болевые ощущения и дискомфорт в области сердца кроме заболеваний сердца могут быть обусловлены патологией других органов грудной клетки (легких, плевры, средостения, диафрагмы), пищеварительного тракта, костно-мышечных структур грудной стенки, заболеваниями нервной системы, а также психогенными состояниями.
На начальном этапе постановки диагноза рекомендуется опираться на клинические проявления (особенности болевого синдрома и менее характерные симптомы и признаки, потенциально связанные с острой ишемией миокарда), данные анамнеза, наличие факторов риска ИБС, характер изменений на ЭКГ, в некоторых случаях — данные о локальной сократительной функции желудочков сердца, а также на оценку времени от последнего болевого эпизода до контакта с врачом. При возможности следует проводить сопоставление ЭКГ, зарегистрированных во время и вне преходящих симптомов и признаков, потенциально связанных с ишемией миокарда, а также до текущего ухудшения клинического течения ИБС.
Дополнительные методы обследования требуются для подтверждения ишемии миокарда при недостаточной информативности ЭКГ, исключения заболеваний со схожей клинической симптоматикой, выявления ИМ и оценки (стратификации) риска неблагоприятного течения заболевания, а также для выявления состояний, влияющих на тактику ведения пациента.
Рекомендуется учитывать догоспитальное использование лекарственных средств, которые могут повлиять на тактику ведения пациента с ОКСбпST.
2.2. Физикальное обследование
- У всех пациентов с подозрением на ОКСбпST рекомендуется физикальное обследование для проведения дифференциальной диагностики, выявления признаков, позволяющих оценить наличие и тяжесть других заболеваний, провоцирующих или осложняющих течение ОКСбпST и способных повлиять на выбор подходов к лечению, а также для оценки наличия осложнений ОКСбпST [3][12-14].
ЕОК IС (УУР С, УДД 5)
Комментарии. Основная задача физикального обследования у пациентов с ОКСбпST — выявление состояний, указывающих на высокий риск неблагоприятного госпитального или отдаленного прогноза. К ним можно отнести проявления декомпенсации СН, аускультативные шумы при наличии сопутствующих пороков сердца или механических осложнениях ИМ, признаки, позволяющие заподозрить тромбоэмболию легочной артерии (ТЭЛА) или острый аортальный синдром, проявления заболеваний желудочно-кишечного тракта, центральной нервной системы, патологии почек.
2.3. Лабораторные диагностические исследования
- У всех пациентов с подозрением на ОКСбпST рекомендуется исследование динамики уровня сердечного тропонина Т или I (согласно номенклатуре медицинских услуг: исследование уровня тропонинов I, T в крови; при невозможности их выполнения — экспресс-исследование уровня тропонинов I, T в крови), для подтверждения/исключения ИМ и оценки риска неблагоприятного исхода, с получением результата в течение 60 мин после взятия крови [15-19].
ЕОК IB (УУР A, УДД 1)
Комментарии. Высокочувствительные методы определения (т. е. позволяющие обнаружить сердечный тропонин у ~50-95% здоровых людей), выполненные на автоматических анализаторах, обеспечивают более высокую диагностическую точность по сравнению с тестами в формате исследований по месту оказания медицинской помощи (point of care, POC). Большинство тестов РОС не могут считаться высокочувствительными, демонстрируют более низкую диагностическую точность и прогностическое значение отрицательного результата. Однако они имеют преимущество в быстром получении результата. Рандомизированное исследование с участием пациентов низкого риска с подозрением на острый ИМ и появлением симптомов за ≥2 ч до вызова скорой помощи показало, что использование стратегии исключения острого ИМ на догоспитальном этапе (однократное измерение тропонина стандартным РОС-тестом) приводило к значительному снижению 30-дневных затрат на лечение и имело сопоставимую частоту серьезных неблагоприятных сосудистых событий при сравнении со стратегией исключения при стандартном протоколе в отделении неотложной помощи [20].
В целом автоматизированные методы исследования высокочувствительного тропонина предпочтительны, однако быстро развивающаяся система РОС-тестирования в ближайшее время заставит переоценить это предпочтение, когда станут клинически доступны более тщательно проверенные высокочувствительные тесты POC [3].
Оценка уровня сердечных тропонинов в крови является более чувствительным и специфичным методом, чем определение МВ-фракции креатинфосфокиназы или миоглобина. Последний не рекомендуется для использования.
Рекомендуется использовать количественное определение концентрации сердечных тропонинов в крови, предпочтительно высокочувствительными методами. Качественные и полуколичественные методики могут использоваться при недоступности количественного метода, однако они непригодны для выявления динамики концентрации сердечного тропонина в крови. Преходящее повышение уровня сердечного тропонина в крови свидетельствует об остром повреждении кардиомиоцитов вне зависимости от причины, которая может быть связана как с первичным ограничением коронарного кровотока, так и другими, в т. ч. внесердечными, факторами (Приложение А3. Причины повышения концентрации сердечного тропонина в крови) [5]. Повышение уровня сердечного тропонина выше 99-го перцентиля верхней референсной границы в условиях, указывающих на наличие ишемии миокарда, свидетельствует об остром ИМ (Приложение А3. Причины повышения концентрации сердечного тропонина в крови). У пациентов с нарушенной фильтрационной функцией почек повышенная концентрация сердечного тропонина в крови часто связана с неишемическим повреждением кардиомиоцитов и свидетельствует о повышенном риске сердечных осложнений [19].
Остро возникшее повышение концентрации сердечного тропонина в крови наблюдается не только у пациентов с ИМ 1 типа, но и при патогенетических вариантах ИМ, не связанных с атеротромбозом, а также при остром неишемическом повреждении миокарда при множестве других заболеваний и состояний (ОСН и хроническая СН (ХСН), ТЭЛА, миокардиты, кардиомиопатии, острый аортальный синдром, сепсис, острое нарушение мозгового кровообращения, отравления и т. д.).
- В ранние сроки после госпитализации пациентам с ОКСбпST рекомендуется использовать валидизированные алгоритмы, предполагающие определение концентрации сердечных тропонинов T или I в крови высокочувствительными методами (согласно номенклатуре медицинских услуг: исследование уровня тропонинов I, T в крови) для быстрого подтверждения или исключения ИМ (повторное взятие крови через 1 ч или 2 ч, или 3 ч — в зависимости от используемого алгоритма 0/1 или 0/2, или 0/3 часа) [21-43].
ЕОК IB (УУР A, УДД 2)
Комментарии. Повышение концентрации сердечных тропонинов в крови до диагностически значимых уровней происходит в период от 1 до 6 ч после эпизода ишемии миокарда в зависимости от чувствительности метода. Поэтому часто требуется повторная оценка уровня этого показателя для выявления диагностически значимой динамики, свидетельствующей в пользу развития острого повреждения миокарда. При ОКСбпST изменение уровня биомаркеров в крови используется как для выявления острого ИМ, так и для стратификации риска неблагоприятного исхода и принятия решения по стратегии ведения пациента — выбора инвазивного или неинвазивного подходов к лечению, определения времени выполнения КГ.
Использование высокочувствительных методов определения концентрации сердечных тропонинов в крови предпочтительнее, поскольку они обладают большей диагностической точностью. Для высокочувствительных методов определения сердечного тропонина в крови для выявления острого ИМ рекомендуются протоколы с его повторным определением через 1 ч, 2 ч (Приложение А3. Алгоритм исключения и подтверждения острого повреждения миокарда с учетом уровней сердечного тропонина в крови, определенных высокочувствительными методами при госпитализации и через 1 ч или 2 ч) [37-42] или 3 ч (Приложение А3. Алгоритм исключения и подтверждения острого повреждения миокарда с учетом уровней сердечного тропонина в крови, определенных высокочувствительными методами при госпитализации и через 3 ч) [42][43] при условии, что применяемый диагностикум валидирован в рамках указанных алгоритмов и для него известны пороговые значения показателей. При анализе крови в первый час после начала боли повторную оценку уровня сердечного тропонина высокочувствительным методом рекомендуется проводить через 3 ч.
У пациентов с нормальным уровнем сердечного тропонина при двукратном определении и сохраняющемся клиническом подозрении на ОКС следует предусмотреть дополнительные определения в более поздние сроки заболевания (через 3 ч и иногда позже, особенно при использовании невысокочувствительных методов определения концентрации сердечного тропонина в крови).
- У всех пациентов с подозрением на ОКСбпST рекомендуется проведение общего (клинического) анализа крови, анализа крови биохимического общетерапевтического (общий белок, мочевина, общий билирубин, определение активности аспартатаминотрансферазы и аланинаминотрансферазы в крови), общего (клинического) анализа мочи в рамках первичного обследования при поступлении в стационар и, при необходимости, последующего динамического наблюдения во время госпитализации [3][12][15-17].
ЕОК 1C (УУР C, УДД 5)
Комментарии. Выявление сниженного уровня общего гемоглобина, эритроцитов и/или тромбоцитов в крови является признаком повышенного геморрагического риска; при высоком уровне тромбоцитов повышен риск раннего тромбоза стента. В период госпитализации лабораторные исследования следует повторять при наличии клинической необходимости.
- Всем пациентам с подозрением на ОКСбпST для оценки риска ишемических и геморрагических событий, а также для обеспечения безопасности лечения рекомендуется исследование уровня креатинина в крови при поступлении в стационар и далее при наличии клинической необходимости с расчетом скорости клубочковой фильтрации, а в случае назначения прямых оральных антикоагулянтов еще клиренса креатинина [44-46].
ЕОК IA (УУР A, УДД 3)
Комментарии. Уровень креатинина в крови и расчетный показатель состояния фильтрационной функции почек важны для выбора дозы ряда лекарственных средств (выбор критерия почечной функции определяется Инструкцией к применению лекарственного средства). Расчетная скорость клубочковой фильтрации (рСКФ) используется в диагностике хронической болезни почек (ХБП) и важна для оценки риска ишемических и геморрагических событий.
- У всех пациентов с подозрением на ОКСбпST рекомендуется исследование уровня глюкозы в крови натощак, скрининг на наличие сахарного диабета (СД) (исследование обмена глюкозы; проведение глюкозотолерантного теста; исследование уровня гликированного гемоглобина в крови), а также повторное исследование уровня глюкозы в крови при СД в анамнезе или гипергликемии при госпитализации с целью оценки риска неблагоприятного исхода [47-50].
ЕОК IIaB (УУР В, УДД 3)
Комментарии. Уровень глюкозы в крови рекомендуется также для определения показаний к использованию сахароснижающих лекарственных средств и выбора их дозы. В период госпитализации оценку концентрации глюкозы в крови следует повторять при наличии клинической необходимости.
- Пациентам с ОКСбпST как можно быстрее после госпитализации, предпочтительно в первые 24 ч, рекомендуется выполнить анализ крови для оценки нарушений липидного обмена биохимический (исследование уровня холестерина (ХС) крови, уровня ХС липопротеидов низкой плотности (ЛНП), уровня ХС липопротеидов высокой плотности (ЛВП) и триглицеридов) для последующего сопоставления с уровнями липидов, достигнутыми при использовании гиполипидемических лекарственных средств [12][51].
ЕОК IC (УУР C, УДД 5)
Комментарии. Необходимо определить как минимум уровень ХС, ХС ЛНП, ХС ЛВП и триглицеридов.
- У всех пациентов с ОКСбпST для обеспечения безопасности лечения рекомендуется определение концентрации электролитов крови (минимально — исследование уровня калия в крови, исследование уровня натрия в крови, оптимально — исследование уровня калия в крови, исследование уровня натрия в крови, исследование уровня общего магния в сыворотке крови) и, при наличии клинической необходимости, исследование кислотно-основного состояния и газов крови с коррекцией и повторной оценкой при наличии отклонений от нормальных величин [52-54].
ЕОК IIbС (УУР С, УДД 4)
Комментарии. В период госпитализации оценку концентрации электролитов в крови следует повторять при наличии клинической необходимости.
- Всем пациентам с ОКСбпST, поступающим в стационар, рекомендуется выполнение коагулограммы (активированное частичное тромбопластиновое время (АЧТВ), протромбиновое время, протромбиновый индекс, фибриноген), определение международного нормализованного отношения (МНО), в случае приема антагониста витамина К, а также для прогноза риска периоперационных кровотечений и величины кровопотери — резус фактора, определение основных групп по системе AB0, определение антигена D системы Резус (резус-фактор), определение фенотипа по антигенам C, c, E, e, Cw, K, k. Определение антигена (HbsAg) вируса гепатита B (Hepatitis B virus) в крови, определение антител к вирусу гепатита C (Hepatitis C virus) в крови, определение антител к бледной трепонеме (Treponema pallidum) в крови, определение антител классов M, G (IgM, IgG) к вирусу иммунодефицита человека ВИЧ-1 (Human immunodeficiency virus HIV 1) в крови, определение антител классов M, G (IgM, IgG) к вирусу иммунодефицита человека ВИЧ-2 (Human immunodeficiency virus HIV 2) в крови всем пациентам с ОКСбпST для исключения ассоциации с ВИЧ-инфекцией, гепатитом [3][55-63].
РКО 1C (УУР C, УДД 4)
2.4. Инструментальные диагностические исследования
- В течение 10 мин от начала первичного медицинского контакта с пациентом с подозрением на ОКС для определения типа ОКС и дифференциальной диагностики с иными заболеваниями рекомендуется регистрация ЭКГ в 12 отведениях на месте первичного контакта и интерпретация ЭхоКГ-данных квалифицированным специалистом (медицинским работником) [3][64-71].
ЕОК IB (УУР С, УДД 4)
Комментарии. Если у медицинского персонала первичного медицинского контакта возникают сложности с интерпретацией ЭКГ на месте регистрации, рекомендуется расшифровка, описание и интерпретация ЭКГ-данных с применением телемедицинских технологий. Повторная регистрация ЭКГ на этапе первого медицинского контакта может быть целесообразна в случае изменения характера болевого синдрома или клиники ОКС.
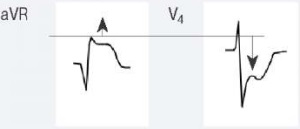
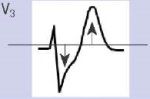
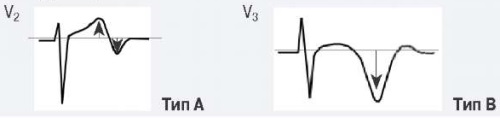
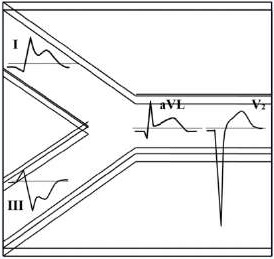
Для ОКСбпST характерно наличие следующих ЭКГ-признаков ишемии миокарда: (1) преходящий (продолжительностью <20 мин) подъем сегмента ST как минимум в двух смежных отведениях; (2) преходящая или стойкая депрессия сегмента ST (особенно горизонтальная или косонисходящая) ≥0,05 Мв как минимум в двух смежных отведениях; (3) инверсия зубца Т >0,1 мВ как минимум в двух смежных отведениях; выраженные (≥0,2 мВ) симметричные отрицательные зубцы Т в прекардиальных отведениях с высокой долей вероятности предполагают наличие острой ишемии миокарда. К неспецифическим признакам относят смещение сегмента ST <0,05 мВ и инверсию зубца Т <0,1 мВ.
Рекомендуется обращать внимание также на другие изменения ЭКГ, способные повлиять на подходы к лечению пациента (Приложение А3. Изменения ЭКГ, влияющие на лечение пациента с ОКСбпST).
Если изменения в 12 стандартных отведениях ЭКГ неинформативны, а по клиническим данным предполагается наличие ишемии миокарда, рекомендуется использовать дополнительные отведения, такие как V7-V9, V3R-V4R [68][69]. При неинформативной ЭКГ у пациентов с сохраняющимся подозрением на ОКС, продолжающимися или возобновляющимися симптомами, для своевременного выявления ишемических изменений на ЭКГ рекомендуется регистрировать повторно (например, с интервалами в 15-30 мин в течение первого часа) или начать дистанционное наблюдение за ЭКГ-данными (мониторирование ЭКГ) с оценкой смещения сегмента ST в 12 отведениях [70].
Отсутствие ишемических изменений на ЭКГ не должно исключать диагноз ОКСбпST. Важный диагностический прием — сравнение с ЭКГ, зарегистрированной до наступления настоящего приступа.
- У всех пациентов с подозрением на ОКСбпST рекомендуется скорейшее начало дистанционного наблюдения за электрокардиографическими данными (мониторирования электрокардиографических данных) до тех пор, пока диагноз не будет исключен, для своевременного выявления опасных нарушений сердечного ритма [3][72].
ЕОК IC (УУР C, УДД 5)
Комментарии. У пациентов с продолжающимися или возобновляющимися симптомами или признаками, предположительно связанными с ишемией миокарда (прежде всего — болевым синдромом), рекомендуется дистанционное наблюдение за ЭКГ-данными (мониторирование ЭКГ) с оценкой смещения сегмента ST в 12 отведениях, если это технически возможно [70].
- У пациентов с ОКСбпST для своевременного выявления опасных нарушений сердечного ритма и/или проводимости рекомендуется дистанционное наблюдение за ЭКГ-данными (мониторирование ЭКГ-данных) длительностью до 24 ч или успешного выполнения ЧКВ, или >24 ч при высоком риске возникновения желудочковой тахикардии (ЖТ) и/или ФЖ [3][72][73].
ЕОК IC (УУР C, УДД 5)
Комментарии. Риск возникновения ЖТ и/или ФЖ повышен при ранее выявленных угрожающих жизни нарушениях сердечного ритма; сохраняющейся или возобновляющейся ишемии миокарда; сумме баллов по шкале GRACE 1.0 >140, фракции выброса (ФВ) ЛЖ <40% (по некоторым данным <35%), в особенности при их сочетании; ОСН, связанной с ишемией миокарда; механических осложнениях ИМ; неоптимальном результате реперфузионной терапии и осложнениями при ее выполнении; сохранении критических стенозов в КА, помимо симптом-связанной КА [74-76].
- У пациентов c НС рекомендуется дистанционное наблюдение за электрокардиографическими данными (мониторирование ЭКГ-данных), если риск возникновения ЖТ и/или ФЖ высокий или предполагается вазоспастический характер ишемии миокарда, для своевременного выявления опасных нарушений сердечного ритма [3][72][73].
ЕОК IIbC (УУР C, УДД 5)
- При подозрении на ОКСбпST у части пациентов рекомендуется выполнение КГ для уточнения диагноза, оценки прогноза, определения показаний для инвазивного лечения и выбора метода реваскуляризации миокарда. Целесообразность КГ и срочность ее выполнения при ОКСбпST определяется клинической картиной заболевания и результатом стратификации риска неблагоприятного исхода [77][78].
ЕОК IA (УУР В, УДД 1)
Комментарии. Основная задача КГ — определение показаний для инвазивного лечения и выбор метода реваскуляризации миокарда. КГ может оказаться полезной (1) для выявления КА и/или ее участка, ответственного за развитие ОКС, (2) для подтверждения диагноза ОКС (обнаружение окклюзирующего или пристеночного тромбоза КА) или его исключения (обнаружение интактных КА становится поводом для поиска альтернативных причин боли в грудной клетке) с возможностью избежать ненужного в этом случае антитромботического лечения, (3) для оценки ближайшего и отдаленного прогноза, особенно у пациентов, не подвергнутых реваскуляризации.
Если данных КГ недостаточно, для уточнения характера и локализации патологического изменения в КА рекомендуются методы внутрисосудистой визуализации (внутрисосудистое ультразвуковое исследование (ВСУЗИ) сосудистой стенки, оптическая когерентная томография (ОКТ) КА) [3][79-84]. Для уточнения функциональной значимости стенозов КА рекомендуется измерение фракционного резерва кровотока (ФРК) или моментального резерва кровотока [85-88].
- Всем пациентам с подозрением на ОКСбпST рекомендуется выполнить трансторакальную ЭхоКГ с обязательной оценкой ФВ ЛЖ для уточнения диагноза, подходов к лечению, проведения дифференциальной диагностики и выявления осложнений [3][89-94].
ЕОК IC (УУР C, УДД 5)
Комментарии. ЭхоКГ (как минимум ультразвуковое исследование (УЗИ)-ассистированное обследование) желательно выполнить до инвазивного обследования, однако при наличии показаний для экстренного вмешательства ее выполнение не должно задерживать транспортировку пациента на КГ. Экстренное выполнение ЭхоКГ рекомендуется пациентам с ОСН, при подозрении на механические осложнения ИМ (острая митральная регургитация на фоне дисфункции папиллярных мышц, отрыв папиллярных мышц или хорд створок митрального клапана, разрыв МЖП, разрыв свободной стенки ЛЖ), расслоение восходящего отдела аорты, тампонаду сердца, дисфункцию клапанов сердца (аортальный стеноз, митральная недостаточность), а также при неинформативной ЭКГ (блокада ЛНПГ, ритм ЭКС и др.). Дополнительное УЗИ легких и нижней полой вены (дуплексное сканирование нижней полой вены и вен портальной системы) позволяет выявить субклинические признаки застоя (появления СН).
У пациентов в критическом состоянии целесообразно использовать формализованный краткий протокол ЭхоКГ (как минимум УЗИ-ассистированное обследование) для скрининга основных возможных причин нарушения гемодинамики. ЭхоКГ необходима для оценки функции и геометрии ЛЖ (с выявлением признаков, предполагающих наличие ишемии или некроза миокарда), а также для распознавания таких осложнений, как тромбоз в полостях сердца, разрывы сердца, нарушение функции клапанов сердца, ИМ ПЖ, перикардит. Существенна роль ЭхоКГ в диагностике синдрома такоцубо. Оценка динамики локальной и глобальной функции ЛЖ помогает уточнить эффективность лечения. Кроме того, ЭхоКГ необходима для определения прогноза заболевания и оценки необходимости использования лекарственных средств с положительным влиянием на прогноз [91-93]. В некоторых случаях проведение ЭхоКГ позволяет уточнить локализацию связанного с данным обострением ИБС поражения коронарного русла.
- При необходимости проведения дифференциальной диагностики у пациентов с подозрением на ОКСбпST, выявления сопутствующих заболеваний и/или осложнений рекомендуется выполнить обзорную рентгенографию органов грудной клетки [95][96].
ЕОК IC (УУР С, УДД 4)
Комментарии. Если выполняется КТ органов грудной полости, обзорная рентгенография органов грудной клетки не требуется.
- МРТ сердца с контрастированием при ОКСбпST рекомендуется рассмотреть в качестве дополнительного метода для уточнения локализации и объема поражения миокарда при относительно небольших его размерах, а также для дифференциальной диагностики поражений миокарда — при наличии технической возможности [5][97][98].
ЕОК IC (УУР С, УДД 5)
Комментарии. МРТ сердца с контрастированием не рекомендуется в качестве рутинного метода обследования пациентов с ОКСбпST. Она позволяет подтвердить наличие очага некроза в миокарде, дифференцировать ишемическую или воспалительную (миокардит) природу поражения, подтвердить или отвергнуть наличие синдрома такоцубо, является эталонным методом трехмерной оценки морфологии и функции камер сердца, а также клапанного аппарата. Дополнительное преимущество метода — отсутствие лучевой нагрузки. Однако технические сложности выполнения ограничивают ее применение в ранние сроки госпитализации.
- МРТ сердца рекомендуется в случае неадекватной визуализации верхушки ЛЖ другими методами у пациентов с высокой вероятностью наличия тромба в ЛЖ — при наличии технической возможности [99][100].
ЕОК IIаС (УУР C, УДД 4)
- Для выявления ишемии миокарда у пациентов с подозрением на ОКСбпST без возобновляющихся приступов боли в грудной клетке, без ишемических изменений на ЭКГ в динамике и без диагностически значимого повышения уровня сердечного тропонина в крови при повторных определениях (предпочтительно высокочувствительным методом) рекомендуется выполнение неинвазивного стресс-теста, при необходимости — повторно (см. Термины и определения) [101-107].
ЕОК IIaА (УУР A, УДД 2)
Комментарии. Неинвазивные стресс-тесты с визуализацией сердца предпочтительнее нагрузочной пробы под контролем ЭКГ и не имеют альтернативы в случаях, когда исходные изменения на ЭКГ препятствуют выявлению ишемии. Предпочтительно выполнение неинвазивных стресс-тестов в первые 72 ч после госпитализации, если нет противопоказаний.
В отдельных случаях для выявления ишемии миокарда при наличии технической возможности возможно использование сцинтиграфии миокарда [108][109].
- КТ КГ рекомендуется для исключения ОКС у пациентов с невысокой вероятностью наличия ИБС при отсутствии ишемических изменений на ЭКГ и повышенного уровня сердечного тропонина Т или I в крови [110-116].
ЕОК IIaA (УУР A, УДД 1)
Комментарии. КТ КГ не рекомендуется в качестве рутинного метода обследования при ОКСбпST. Она не должна использоваться для исключения ОКС у пациентов с известным коронарным атеросклерозом и имеет ограничения при выраженной кальцификации КА, тахикардии, нерегулярной частоте сердечных сокращений (ЧСС). Значение этого метода у пациентов с имплантированным коронарным стентом или перенесших операцию КШ не определено. Его целесообразность при использовании высокочувствительных методов определения концентрации сердечного тропонина в крови не ясна.
- При необходимости дифференциальной диагностики ОКСбпST с другими заболеваниями (расслоение аорты, ТЭЛА, пневмоторакс, плеврит и пр.) рекомендуется КТ органов грудной полости [12][117].
РКО IC (УУР С, УДД 5)
2.5. Иные диагностические исследования
2.5.1. Стратификация риска неблагоприятного исхода
- Для стратификации риска неблагоприятного исхода и выбора стратегии ведения у пациентов с ОКСбпST рекомендуется осуществлять совокупную оценку анамнеза, клинических данных, ЭКГ, ЭхоКГ, результатов исследования уровня сердечного тропонина Т или I в крови (предпочтительно методом с высокой чувствительностью), фильтрационной функции почек (рСКФ) и, в некоторых случаях, визуализирующих методик выявления ишемии миокарда [15][66][118-124].
ЕОК IA (УУР С, УДД 4)
Комментарии. Группы риска неблагоприятного исхода при ОКСбпST и критерии отнесения пациентов к каждой из этих групп представлены в Приложении А3. Категории риска неблагоприятного исхода при ОКСбпST.
Помимо оценки вероятности неблагоприятного исхода, стратификация риска необходима для выделения пациентов, которым показана экстренная КГ, тех, кому КГ должна быть выполнена в первые 24 ч после госпитализации (или обращения пациента за помощью, если ОКСбпST развился в стационаре), и тех, кому для определения целесообразности выполнения КГ требуется учет реакции на первоначальное медикаментозное лечение и проведение дополнительного обследования.
Кроме того, у пациентов с ОКСбпST следует учитывать изменения на ЭКГ, которые могут свидетельствовать об окклюзионном характере поражения КА (Приложение А3. Изменения ЭКГ, влияющие на лечение пациента с ОКСбпST). Результаты КГ также являются основанием для уточнения степени риска неблагоприятного исхода.
- У пациентов с ОКСбпST для стратификации риска неблагоприятного исхода рекомендуется использовать валидизированные индексы и шкалы, в частности шкалу GRACE [118][120][124-135].
ЕОК IIaB (УУР А, УДД 2)
Комментарии. Для оценки прогноза при ОКСбпST (Приложение А3. Категории риска неблагоприятного исхода при ОКСбпST) рекомендуется использовать шкалу GRACE (Приложение Г1. Оценка риска неблагоприятного исхода при ОКСбпST с использованием шкалы GRACE) [118][126-131][134]. При выборе подходов к ведению пациентов с ОКСбпST рекомендуется использовать шкалу GRACE 1.0. Один из ее вариантов дает возможность осуществить стратификацию риска при госпитализации, другой — при выписке из стационара.
В качестве инструментов индивидуальной оценки риска кровотечений при ОКСбпST возможно использование шкалы ARC-HBR (Приложение Г2) [135]. В дополнение к ней могут применяться шкала CRUSADE (оценка риска крупных кровотечений в период госпитализации у пациентов, подвергнутых КГ) (Приложение Г3) [45] и шкала PRECISE-DAPT (оценка риска кровотечений у стентированных пациентов при использовании двойной антитромбоцитарной терапии) [133] (Приложение Г4). Имеется также отечественная шкала оценки риска кровотечений ОРАКУЛ, применимая в т. ч. при использовании сочетания антиагрегантов с антикоагулянтами [46][136-138] (Приложение Г5).
У пациентов с высоким риском кровотечений рекомендуются мероприятия по его снижению. Высокий риск кровотечений не должен автоматически приводить к отказу от наиболее эффективных способов антитромботического и инвазивного лечения ОКСбпST. Выбор подходов к лечению пациента с высоким риском кровотечений должен быть персонифицирован с учетом опасности тромботических осложнений, возможности контроля потенциальных источников кровотечений, данных о соотношении пользы и риска у каждого из планируемых вмешательств, а также всех других обстоятельств ведения конкретного пациента.
3. Лечение, включая медикаментозную и немедикаментозную терапию, диетотерапию, обезболивание, медицинские показания и противопоказания к применению методов лечения
3.1. Инвазивное лечение заболевания
3.1.1. Выбор стратегии лечения пациентов с ОКСбпST в стационаре
- У пациентов с ОКСбпST для улучшения прогноза и контроля симптомов заболевания на основании оценки риска неблагоприятного исхода рекомендуется выбрать и реализовать одну из инвазивных стратегий лечения в стационаре: КГ с намерением выполнить реваскуляризацию миокарда (ЧКВ или операция КШ) [77][139-148].
ЕОК IA (УУР A, УДД 1)
Комментарии. Рекомендуются следующие стратегии инвазивного лечения пациентов с ОКСбпST:
— немедленная инвазивная (КГ с намерением выполнить реваскуляризацию миокарда в первые 2 ч после госпитализации),
— ранняя инвазивная (КГ с намерением выполнить реваскуляризацию миокарда в первые 24 ч после госпитализации),
— селективная (избирательная) инвазивная стратегия (первоначальное медикаментозное лечение, КГ с намерением выполнить реваскуляризацию миокарда только при возобновлении ишемии миокарда, появлении осложнений, а также у пациентов с высокой вероятностью наличия ОКСбпST).
Выбор стратегии основан на наличии критериев высокого и очень высокого риска неблагоприятного исхода (Приложение А3. Категории риска неблагоприятного исхода при ОКСбпST).
- У пациентов с ОКСбпST и хотя бы одним критерием очень высокого риска рекомендуется проведение КГ с намерением выполнить реваскуляризацию миокарда в максимально ранние сроки после госпитализации (первые 2 ч) с целью снижения вероятности неблагоприятных исходов [3][149].
ЕОК IC (УУР C, УДД 5)
Комментарии. Критерии очень высокого риска неблагоприятного исхода: нестабильность гемодинамики, кардиогенный шок; продолжающаяся или повторяющаяся боль в грудной клетке, рефрактерная к медикаментозному лечению; угрожающие жизни аритмии или остановка кровообращения; ОСН, предположительно связанная с сохраняющейся ишемией миокарда; механические осложнения острого ИМ (разрыв свободной стенки ЛЖ, разрыв МЖП, разрыв папиллярных мышц или хорд створок митрального клапана); повторные динамические смещения (особенно подъем) сегмента ST или изменения зубца T, предполагающие наличие ишемии миокарда. Неотложная инвазивная стратегия должна быть реализована у таких пациентов независимо от уровня биомаркеров некроза миокарда в крови, изменений на ЭКГ и количества баллов по различным шкалам риска. Стационары без возможности выполнить экстренное ЧКВ должны немедленно переводить таких пациентов в инвазивные центры.
Если ОКСбпST развился в стационаре, отсчет времени до КГ рекомендуется начать с момента обращения пациента за помощью.
- У пациентов с ОКСбпST, относящихся к категории высокого риска, рекомендуется проведение КГ с намерением выполнить реваскуляризацию миокарда до выписки из стационара для снижения вероятности неблагоприятных исходов [77][140-146][148][150-160].
ЕОК IA (УУР A, УДД 1)
Комментарии. Критерии высокого риска неблагоприятного исхода: наличие ИМбпST; преходящий подъем сегмента ST; динамическое смещение сегмента ST или изменения зубца T; сумма баллов по шкале GRACE 1.0 >140 [140][146][151]. Стационары без возможностей для выполнения ЧКВ должны переводить таких пациентов в инвазивные центры.
Если ОКСбпST развился в стационаре, отсчет времени до КГ рекомендуется начать с момента обращения пациента за помощью.
- У пациентов с ОКСбпST, относящихся к категории высокого риска, рекомендуется проведение КГ с намерением выполнить реваскуляризацию миокарда в первые 24 ч после госпитализации для снижения вероятности рецидива ишемии миокарда и сокращения длительности госпитализации [77][140-146][150-154].
ЕОК IIaA (УУР A, УДД 1)
Комментарии. К критериям высокого риска относятся: наличие ИМбпST; преходящий подъем сегмента ST; динамическое смещение сегмента ST или изменения зубца T; сумма баллов по шкале GRACE 1.0 >140 [140,][146][151]. Инвазивная стратегия в первые 24 ч после госпитализации у таких пациентов не влияет на смертность и частоту ИМ, но приводит к достоверному снижению частоты возобновления ишемии миокарда [154]. Стационары без возможностей для выполнения ЧКВ должны срочно переводить таких пациентов в инвазивные центры. Если КГ с намерением выполнить реваскуляризацию миокарда не была выполнена в первые 24 ч после госпитализации, инвазивная стратегия лечения пациента должна быть реализована во время текущей госпитализации.
Если ОКСбпST развился в стационаре, отсчет времени до КГ рекомендуется начать с момента обращения пациента за помощью.
- У пациентов с ОКСбпST без повторения ишемии миокарда, без критериев очень высокого и высокого риска неблагоприятного исхода решение о целесообразности КГ до выписки из стационара и реваскуляризации миокарда по ее итогам рекомендуется принимать на основании клинической картины заболевания и результатов дополнительного обследования [77][78][102][139-149][152][153][155-162].
ЕОК IA (УУР A, УДД 1)
Комментарии. К дополнительным обследованиям относятся: КТ КГ и неинвазивные стресс-тесты (предпочтительно с визуализацией миокарда или оценкой его сократимости). У пациентов без повторения ишемии миокарда, без критериев очень высокого и высокого рисков неблагоприятного исхода при высокой вероятности наличия ОКСбпST предпочтительна КГ до выписки из стационара с решением вопроса о целесообразности реваскуляризации миокарда.
- Во всех медицинских учреждениях, оказывающих помощь пациентам с ОКСбпST, рекомендуется фиксировать и контролировать указанные в данном документе временные интервалы выполнения инвазивного лечения [3].
ЕОК IС (УУР С, УДД 5)
3.1.2. Способы инвазивного лечения пациентов с ОКСбпST
- У большинства пациентов с ОКСбпST и однососудистым поражением КА рекомендуется выполнять ЧКВ на инфаркт/симптом-связанном стенозе (окклюзии) сразу после КГ с целью снижения риска развития повторных коронарных событий [3].
ЕОК IВ (УУР С, УДД 5)
- У пациентов с ОКСбпST и многососудистым поражением КА при отсутствии кардиогенного шока с целью профилактики повторных ишемических событий рекомендуется рассмотреть возможность полной реваскуляризации в рамках одного вмешательства, в зависимости от технических возможностей, анатомической протяженности поражения коронарного русла и клинического состояния пациента [163][164].
ЕОК IIaC (УУР В, УДД 2)
- У пациентов с ОКСбпST и многососудистым поражением КА выбор между ЧКВ и операцией КШ рекомендуется осуществлять с учетом коронарной анатомии, тяжести состояния пациента и наличия сопутствующих заболеваний, с возможным использованием шкалы SYNTAX. Решение рекомендуется принимать коллегиально с участием кардиолога, кардиохирурга и специалиста по рентгенэндоваскулярным методам диагностики и лечения [149][159].
ЕОК IВ (УУР C, УДД 5)
- При выполнении КГ и ЧКВ у пациентов с ОКСбпST в качестве предпочтительного сосудистого доступа рекомендуется доступ через лучевую артерию с целью снижения риска развития кровотечений, смерти, крупных сердечно-сосудистых осложнений и осложнений в месте пункции — при условии освоенности этого доступа в данном учреждении [165][166].
ЕОК IA (УУР A, УДД 1)
- Для снижения риска повторных коронарных событий и необходимости в повторной реваскуляризации у пациентов с ОКСбпST при первичном ЧКВ рекомендуется предпочесть транслюминальную баллонную ангиопластику со стентированием КА изолированной процедуре транслюминальной баллонной ангиопластики (без стентирования) КА [167].
ЕОК IA (УУР A, УДД 1)
Комментарии. Метаанализы не выявили преимущества стентирования КА перед транслюминальной баллонной ангиопластикой по снижению риска смерти. Большая часть доказательной базы, указывающей на преимущество ЧКВ в снижении смертности при ОКС, получена при использовании транслюминальной баллонной ангиопластики (без стентирования). Поэтому не следует воздерживаться от ЧКВ в виде транслюминальной баллонной ангиопластики, ссылаясь на отсутствие подходящих стентов.
В особых случаях поражения инфаркт-связанной КА (коронарная эмболия, эктазированная КА, спонтанная диссекция КА, вмешательство на ранее стентированном сегменте КА, диффузное поражение дистальных сегментов КА) ее реканализация может быть проведена без использования коронарного стентирования.
- При выполнении транслюминальной баллонной ангиопластики со стентированием КА у пациентов с ОКСбпST для снижения риска повторных коронарных событий, тромбоза стента, необходимости в повторной реваскуляризации рекомендуется использовать СВЛ нового поколения, вне зависимости от длительности и состава планируемой антиагрегантной и антикоагулянтной терапии [168-174].
ЕОК IA (УУР A, УДД 1)
- ВСУЗИ или ОКТ КА рекомендуется рассмотреть в качестве вспомогательных инструментов во время ЧКВ у пациентов с ОКСбпST для улучшения непосредственных и отдаленных результатов вмешательства, при наличии технических возможностей [79-84][175][176].
ЕОК IIaA (УУР А, УДД 2)
Комментарии. В ряде крупных метаанализов и рандомизированных исследований внутрисосудистая визуализация (ВСУЗИ, ОКТ) продемонстрировала достоверное влияние на отдаленные результаты стентирования КА с точки зрения профилактики тромбоза и рестеноза стента. В одном из наиболее крупных метаанализов [177], посвященном сравнению отдаленных исходов у пациентов при стентировании КА с использованием различных методов внутрисосудистой визуализации, ВСУЗИ-контроль приводил к достоверному снижению сердечно-сосудистой смертности, частоты повторных ИМ и тромбоза стента. Внутрисосудистая визуализация может быть полезна для определения инфаркт (симптом)-связанного поражения КА при неочевидности ангиографических данных и для оптимизации ЧКВ.
- При выборе объема реваскуляризации миокарда при ЧКВ у пациентов с ОКСбпST и многососудистым поражением КА при отсутствии кардиогенного шока рекомендуется рассмотреть оценку функциональной значимости стенозов с помощью измерения ФРК или моментального резерва кровотока, при наличии технических возможностей [86][87][164][178-180].
ЕОК IIbВ (УУР C, УДД 4)
Комментарии. Измерение ФРК с применением гиперемических агентов, таких как #трифосаденин (внутривенно (в/в) 140 или 180 мкг/кг/мин или интракоронарно 20 или 40 мкг для одной КА) и #папаверин (интракоронарно 12-20 мг для левой КА и 8-20 мг для правой КА) [180-183], и/или моментального резерва кровотока является стандартом оценки функциональной значимости пограничных (40-90%) стенозов КА. В качестве контрольного значения принимается показатель ФРК ≤0,8 (стеноз считается функционально значимым). Для моментального резерва кровотока этот показатель составляет ≤0,89. В ряде исследований продемонстрировал эффективность применения ФРК для оценки функциональной значимости стенозов и у данной категории пациентов. В исследовании FRAME-AMI [179] было продемонстрировано преимущество применения ФРК в снижении частоты общей смертности, повторного ИМ и незапланированной реваскуляризации. Напротив, исследование FLOWER-MI [184] не продемонстрировало преимущества применения ФРК в снижении частоты неблагоприятных сердечно-сосудистых событий. Также в ряде наблюдений было продемонстрировано, что у пациентов с ОКС может происходить неточная функциональная оценка значимости поражений, т. к. восстановление микроциркуляции происходит не ранее чем через 24 ч от начала ОКС [185][186]. Поскольку представленные в настоящее время результаты исследований противоречивы, целесообразность использования ФРК у пациентов с ОКС остается предметом дальнейшего изучения.
3.1.3. Защита почек при ЧКВ
- У пациентов с ОКСбпST и СД рекомендуется принимать во внимание более высокий риск развития контраст-индуцированной нефропатии, что необходимо учитывать при определении показаний к проведению исследований с введением рентгеноконтрастных препаратов, выборе их объема, а также при принятии решения об использовании активной профилактики (гидратация), когда это позволяет состояние пациента [187].
ЕОК IC (УУР С, УДД 4)
Комментарии. Если пациент получает метформин** и/или ингибитор натрийзависимого переносчика глюкозы 2-го типа, после КГ/ЧКВ можно ожидать ухудшения фильтрационной функции почек. Поэтому до КГ/ЧКВ метформин** рекомендуется отменить, а в случаях, когда вмешательства нельзя отсрочить, тщательно мониторировать фильтрационную функцию почек.
- У пациентов с ОКСбпST и ХБП выполнение КГ и ЧКВ рекомендуется после тщательной оценки соотношения риска и пользы, с учетом выраженности нарушения фильтрационной функции почек [187].
ЕОК IB (УУР С, УДД 4)
- У пациентов с умеренной или тяжелой ХБП при инвазивной стратегии лечения ОКСбпST рекомендуется гидратация изотоническим раствором натрия хлорида** и применение низко- или изоосмолярного контрастного средства (в минимальном объеме) для профилактики острого повреждения почек [188-194].
ЕОК IIaB (УУР A, УДД 2)
Комментарии. У пациентов с умеренной или тяжелой ХБП (рСКФ 15-44 мл/мин/1,73 м²) рекомендуется ограничить объем вводимого контрастного вещества (соотношение объема контраста к рСКФ <3,7). Если ожидаемый объем контрастного вещества во время КГ/ЧКВ превышает 100 мл, рекомендуется гидратация с использованием изотонического раствора натрия хлорида** — в/в инфузия со скоростью 1 мл/кг/ч за 12 ч до процедуры (если это возможно) и как минимум 24 ч после ее окончания (для пациентов с ФВ ЛЖ ≤35% или ХСН >2 ФК по NYHA — 0,5 мл/кг/ч). Возможно также использование методик с гидратацией под контролем центрального венозного давления (ЦВД) или в/в введением #фуросемида** с восполнением объема потерянной жидкости изотоническим раствором натрия хлорида**.
У пациентов с тяжелой ХБП (рСКФ 15-29 мл/мин/1,73 м²) может быть рассмотрена целесообразность профилактической гемофильтрации за 6 ч до ЧКВ (если это возможно) с замещением жидкости со скоростью 1000 мл/ч без ее потери и гидратация как минимум 24 ч после процедуры.
3.1.4. Спонтанная диссекция КА
Спонтанная диссекция КА — это неатеросклеротическое поражение КА, характеризующееся отслоением интимы артерии с формированием субинтимальной гематомы и обструкцией просвета артерии спонтанной (неятрогенной) природы. Спонтанная диссекция КА встречается примерно в 4% случаев всех ОКС, преимущественно у женщин младше 60 лет. Существует 3 ангиографических типа спонтанных диссекций КА: 1 тип — наличие нескольких визуальных просветов в КА; 2 тип — наличие протяженного гладкого поражения, формирующегося за счет объемной интрамуральной гематомы; 3 тип — локальный стеноз, который выглядит как АСБ.
Клинические проявления спонтанной диссекции КА не отличаются от таковых при атеротромбозе, в связи с чем первичная стратегия соответствует стандартным рекомендациям по лечению больных с ОКС (в т. ч. с ОКСбпST). Наиболее точными методами для подтверждения спонтанной диссекции КА во время КГ являются внутрисосудистые методы визуализации (ВСУЗИ и ОКТ).
- При полной обструкции КА, обусловленной наличием спонтанной диссекции, при гемодинамической нестабильности пациента, сохранении признаков ишемии, высоком риске сердечно-сосудистых осложнений в качестве оптимальной стратегии реваскуляризации для восстановления коронарного кровотока у пациентов с ОКСбпST рекомендуется стандартное ЧКВ с имплантацией СВЛ [195][196].
ЕОК IC (УУР С, УДД 4)
Комментарии. Стратегии эндоваскулярного лечения пациентов со спонтанными диссекциями КА в настоящее время не определены. Основные технические аспекты эндоваскулярного лечения пациентов со спонтанными диссекциями отражены в консенсусном документе ЕОК, согласно которому для подтверждения спонтанных диссекций (наличия ложного просвета и/или интрамуральной гематомы) как причины обструкции КА следует рассмотреть ВСУЗИ или ОКТ для подтверждения наличия ложного просвета и/или интрамуральной гематомы [196][197]. Внутрисосудистые методы визуализации обладают высоким разрешением и позволяют достоверно установить наличие диссекции, её тип, и определить степень компрометации просвета и позицию интракоронарного проводника (в истинном или ложном просвете).
У пациентов с ОКСбпST со спонтанной диссекцией КА при отсутствии обструкции КА, низком риске повторных событий и отсутствии признаков ишемии, рекомендуется медикаментозная терапия до заживления спонтанной диссекции [196][197].
3.1.5. Инвазивная стратегия лечения у пациентов с кардиогенным шоком и внезапной остановкой кровообращения
- У пациентов с ОКСбпST и кардиогенным шоком для улучшения прогноза рекомендуется скорейшая реваскуляризация миокарда [198].
ЕОК IIaB (УУР В, УДД 2)
Комментарии. Выбор между ЧКВ и операцией КШ в данной клинической ситуации определяется особенностями поражения коронарного русла и наличием механических осложнений ИМ.
- У пациентов с ОКСбпST и кардиогенным шоком рекомендуется выполнение ЧКВ с восстановлением кровотока в инфаркт-связанной КА независимо от времени возникновения симптомов [198][199].
ЕОК IB (УУР B, УДД 2)
- У пациентов с ОКСбпST и кардиогенным шоком из-за возможного увеличения риска смерти и острого почечного повреждения или резкого прогрессирования ХБП рекомендуется воздержаться от одномоментных многососудистых ЧКВ, ограничившись вмешательством на инфаркт-связанной КА [198][199].
ЕОК IIIB (УУР B, УДД 2)
- У пациентов с ОКСбпST и кардиогенным шоком при невозможности выполнения ЧКВ или безуспешном ЧКВ с целью снижения риска смерти рекомендуется неотложное КШ [198][199].
ЕОК IB (УУР B, УДД 2)
Комментарии. В исследовании SHOCK у 302 пациентов с острым ИМ, осложненным кардиогенным шоком, сопоставляли экстренную реваскуляризацию с начальной медицинской стабилизацией. Передний ИМ отмечен у 60% из них, многососудистое поражение КА — в 85% случаев. Среди пациентов, которым была выполнена экстренная реваскуляризация, 64% подверглись ЧКВ, 36% перенесли КШ. Различий в смертности через 30 дней не было (первичная конечная точка), однако через 6 мес. смертность оказалась ниже в группе экстренной реваскуляризации. На основании этих данных пациентам с острым ИМ, осложненным КШ, рекомендуется немедленная КГ и, если возможно, ЧКВ. Пациентам с коронарной анатомией, непригодной для ЧКВ, рекомендуется экстренное КШ.
- У пациентов с ОКСбпST, перенесших кардиогенный шок и индексную процедуру реваскуляризации, при наличии продолжающейся ишемии, нестабильности гемодинамики и с учетом сопутствующих заболеваний с целью улучшения прогноза рекомендуется выполнение этапной реваскуляризации не-инфаркт-связанных КА [3].
ЕОК IIaC (УУР С, УДД 5)
- У гемодинамически стабильных пациентов с ОКСбпST после успешной реанимации по поводу внезапной остановки кровообращения рутинная немедленная КГ не рекомендуется [3][200].
ЕОК IIIA (УУР А, УДД 2)
Комментарии. Тактика ведения пациентов с внезапной остановкой кровообращения должна определяться индивидуально и учитывать состояние пациента, его гемодинамические и неврологические характеристики. Отсутствие преимущества немедленной КГ у пациентов без ишемических изменений на ЭКГ было продемонстрировано в исследовании COACT [200].
3.1.6. ИМ без обструктивного поражения КА
ИМ без обструктивного поражения КА (ИМБОКА) — "рабочий" диагноз, который устанавливается у пациентов с критериями ИМ согласно 4 Универсальному определению [5] (Приложение А3. Критерии диагностики ИМ), когда при КГ ни в одной крупной КА не обнаружено стенозов ≥50%. Термин "ИМБОКА" включает в себя гетерогенную группу патологий, как коронарных, так и некоронарных, в т. ч. некардиальных. Основная цель введения этого термина связана с тем, что пациенту с диагнозом ИМБОКА необходимо проведение дальнейшего обследования для верификации диагноза и проведения адекватного лечения.
- У пациентов с отсутствием обструктивного поражения КА (стенозы ≥50% по данным КГ) при наличии клинических и лабораторных признаков ИМ рекомендован обширный диагностический поиск для определения причины повреждения миокарда и верификации диагноза [179][184][185][201].
ЕОК IС (УУР С, УДД 5)
Комментарии. Диагностический поиск у пациентов с ИМБОКА включает в себя выполнение ЭхоКГ, КГ с применением внутрисосудистых методов визуализации (ВСУЗИ или ОКТ) для исключения пристеночных тромбов, эрозий, спонтанной диссекции КА, тестов для выявления спазма крупных КА и микроциркуляторной обструкции, а также, если необходимо, дополнительных исследований для дифференциальной диагностики ОКС (перечень исследований определяется клиническими рекомендациями по альтернативному(-ым) диагнозу(-ам)).
- С целью этиологического лечения ведение пациентов с ИМБОКА рекомендуется выполнять в соответствии с текущими рекомендациями, касающимися конкретной диагностированной причины ИМБОКА [184][185][201].
ЕОК IB (УУР С, УДД 5)
- При отсутствии очевидной причины ИМБОКА рекомендуется выполнение МРТ сердца с целью уточнения причины повреждения миокарда [177][186][201-203].
ЕОК IВ (УУР В, УДД 3)
3.2. Медикаментозное лечение заболевания
3.2.1. Обезболивание
- Для устранения боли, с целью седации и снижения симпатической активности, приводящей к тахикардии и повышению АД, у пациентов с ОКСбпST рекомендуется в/в введение морфина** (группа N02AA — природные алкалоиды опия; син.: наркотические анальгетики) [204-207].
ЕОК IIaB (УУР С, УДД 4)
Комментарии. У больных с НС в большинстве случаев приступ удается купировать с помощью нитратов. Если ангинозный приступ не ослабевает через несколько минут после прекращения действия провоцирующего фактора (физическая нагрузка) или если он развился в покое, пациенту следует принять нитроглицерин** (группа C01DA — органические нитраты) в дозе 0,4-0,5 мг в виде таблеток под язык или аэрозоля (спрея). Если симптомы не исчезают через 5 мин, а препарат удовлетворительно переносится, можно использовать его повторно. Если боль или дискомфорт в грудной клетке сохраняются в течение 5 мин после повторного приема нитроглицерина, необходимо немедленно вызвать скорую медицинскую помощь. Если 3 приема нитроглицерина** не уменьшают интенсивность приступа, дальнейший прием не имеет смысла. В связи с опасностью артериальной гипотонии постоянно контролируют АД.
Отличительная особенность ангинозного приступа при ИМ (в т. ч. ИМбпST) — слабая реакция или отсутствие реакции на нитроглицерин**. Помимо обезболивания, морфин** способствует уменьшению страха, возбуждения, снижает симпатическую активность, увеличивает тонус блуждающего нерва, уменьшает работу дыхания, вызывает расширение периферических артерий и вен (последнее особенно важно при отеке легких). Доза, необходимая для адекватного обезболивания, зависит от индивидуальной чувствительности, возраста, размеров тела. Перед использованием 10 мг морфина** разводят как минимум в 10 мл 0,9% раствора натрия хлорида** (группа B05BB — растворы, влияющие на водно-электролитный баланс). Первоначально следует ввести в/в медленно 2-4 мг лекарственного вещества. При необходимости введение повторяют каждые 5-15 мин по 2-4 мг до купирования боли или возникновения побочных эффектов, не позволяющих увеличить дозу.
Назначение морфина** приводит к замедлению и ослаблению основного эффекта антиагрегантов (клопидогрел**, тикагрелор**, прасугрел), что может отразиться на результатах лечения у некоторых пациентов [208][209].
При использовании морфина** возможны следующие осложнения:
— выраженная артериальная гипотензия; устраняется в горизонтальном положении в сочетании с поднятием ног (если нет отека легких). Если этого недостаточно, в/в капельно вводится 0,9% раствор натрия хлорида**. В редких случаях — адрено- и допаминстимуляторы (группа C01CA — адренергические и дофаминергические средства);
— выраженная брадикардия в сочетании с артериальной гипотензией; устраняется атропином** (в/в 0,5-1,0 мг);
— тошнота, рвота; устраняются производными фенотиазина, в частности, метоклопрамидом** (в/в однократно 10 мг);
— выраженное угнетение дыхания; устраняется налоксоном** (в/в 0,1-0,2 мг, при необходимости повторно каждые 15 мин), при этом уменьшается и анальгезирующее действие препарата.
Опиаты могут ослаблять перистальтику кишечника и приводить к запорам. Препараты этой группы снижают тонус мочевого пузыря и затрудняют мочевыведение, особенно у мужчин с гипертрофией предстательной железы.
- При наличии признаков выраженного беспокойства и возбуждения для их устранения у пациентов с ОКCбпST рекомендуется назначение транквилизаторов (группа N05BA — производные бензодиазепина) [3][210-212].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. Для уменьшения страха обычно достаточно создать спокойную обстановку и ввести наркотический анальгетик. При выраженном возбуждении могут потребоваться транквилизаторы (например, диазепам** в/в 5-10 мг, для пожилых стартовая доза — 2,5 мг; группа N05BA — производные бензодиазепина). Важное значение для эмоционального комфорта пациента имеют соответствующий стиль поведения персонала, разъяснение диагноза, прогноза и плана лечения. У пациентов с сохраняющимся беспокойством и нарушенным поведением, а также симптомами отмены при никотиновой зависимости, также можно назначить транквилизаторы. При возбуждении и делирии достаточно эффективно и безопасно в/в введение галоперидола**.
3.2.2. Коррекция гипоксемии
- Пациентам с ОКСбпST при наличии гипоксемии (степень насыщения гемоглобина крови кислородом (SpO2) <90% или парциальное давление кислорода в артериальной крови (PaO2) <60 мм рт.cт.) для ее устранения рекомендуется ингаляторное введение кислорода (оксигенотерапия) [3][12].
ЕОК IC (УУР С, УДД 5)
Комментарии. Подача увлажненного кислорода проводится через носовые катетеры со скоростью 2-8 л/мин. Контролируют насыщение крови кислородом, измеряя сатурацию (SpO2) неинвазивно или оценивая показатели газового состава крови (прежде всего, PaO2) лабораторно.
- Из-за отсутствия положительных эффектов на течение болезни ингаляторное введение кислорода (оксигенотерапия) не рекомендуется пациентам с ОКСбпST с уровнем сатурации крови (SpO2) ≥90% [213-217].
ЕОК IIIB (УУР А, УДД 2)
Комментарии. Ингаляторное введение кислорода (оксигенотерапия) не только не приносит пользы пациентам с неосложненным ОКСбпST, но может быть вредно, что предположительно обусловлено увеличением повреждения миокарда.
3.2.3. Антитромботическая терапия
У пациентов с ОКСбпST в начале лечения рекомендуется сочетание ацетилсалициловой кислоты** (АСК**), ингибитора Р2Y12-рецептора тромбоцитов и антикоагулянта с последующим переходом на сочетание АСК** с ингибитором Р2Y12-рецептора тромбоцитов или на сочетание апиксабана**, дабигатрана этексилата**, ривароксабана**, эдоксабана или непрямых антикоагулянтов (антагонистов витамина K) с антиагрегантом [3][218-260][307].
ЕОК IA (УУР A, УДД 1)
Комментарии. Антитромботические препараты и их дозы представлены в Приложении А3. Медикаментозное лечение ОКСбпST и Приложении А3. Дозы антитромботических лекарственных средств при нарушенной функции почек.
Показания к длительному применению антикоагулянтов — ФП/трепетание предсердий (ТП), механический протез клапанов сердца***, тромбоз полости ЛЖ, тромбоз глубоких вен и/или ТЭЛА.
3.2.3.1. Антиагреганты у пациентов, не имеющих показаний к длительному пероральному приему антикоагулянтов
- Для снижения риска смерти, ИМ и ишемического инсульта всем пациентам с ОКСбпST, не имеющим противопоказаний и вне зависимости от стратегии лечения, рекомендуется прием АСК** на неопределенно долгий срок [3][12][218-223].
ЕОК IA (УУР A, УДД 1)
Комментарии. Рекомендуется начальная (нагрузочная) доза АСК** (группа B01AC — антиагреганты кроме гепарина) 150-300 мг (таблетку разжевать и проглотить); постоянная поддерживающая доза АСК** 75-100 мг внутрь 1 раз/сут. со вторых суток лечения пациента [3].
Начало использования АСК** при ОКСбпST на догоспитальном этапе не имеет доказательств эффективности и безопасности (в сравнении с изученным использованием в стационаре).
- Всем пациентам с ОКСбпST, не имеющим высокого риска кровотечений, в добавление к АСК** рекомендуются ингибиторы Р2Y12-рецептора тромбоцитов (клопидогрел**, тикагрелор**, прасугрел) сроком на 12 мес. для снижения суммарного риска смерти, ИМ и ишемического инсульта [3][12][224-229].
ЕОК IA (УУР B, УДД 2)
Комментарии. Начало использования ингибиторов Р2Y12-рецептора тромбоцитов при ОКСбпST на догоспитальном этапе не имеет доказательств эффективности и безопасности (по сравнению с изученным использованием в стационаре).
Досрочное прекращение двойной антиагрегантной терапии увеличивает частоту коронарных осложнений. Частой причиной досрочного прекращения приема одного или обоих компонентов двойной антиагрегантной терапии являются кровотечения и необходимость в хирургическом вмешательстве. В случае необходимости отменить ингибитор Р2Y12-рецептора тромбоцитов из-за хирургического вмешательства крайне желательно реализовать это вмешательство в условиях многопрофильного стационара с возможностью проведения ЧКВ в случае возникновения периоперационного тромбоза стента и ИМ.
При необходимости экстренной хирургической операции с высоким риском кровотечения или серьезном кровотечении лечение ингибитором Р2Y12-рецептора тромбоцитов следует прекратить и возобновить при первой возможности после устранения причин кровотечения.
Тикагрелор** следует отменить как минимум за 3 дня, клопидогрел** — как минимум за 5 дней, прасугрел — как минимум за 7 дней до планового хирургического вмешательства. По мере возможности АСК** следует продолжить, т. к. отмена обоих препаратов еще больше повышает риск коронарного тромбоза. При отсутствии возможности выдержать указанные сроки решение об оперативном лечении следует принимать консилиумом врачей разных специальностей, который должен оценить риски кровотечения и отмены двойной антитромбоцитарной терапии, а также учесть тип хирургического вмешательства, риск рецидива ишемии миокарда, степень поражения КА, время, прошедшее от начала ОКСбпST и ЧКВ, характеристику установленных стентов. При хирургических вмешательствах с низким риском кровотечения не следует досрочно прерывать двойную антитромбоцитарную терапию.
- Если двойная антитромбоцитарная терапия (ACK** в сочетании с ингибитором Р2Y12-рецептора тромбоцитов) у пациента с ОКСбпST была отложена или прервана из-за операции КШ, рекомендуется ее начать или возобновить после операции КШ не менее, чем на 12 мес., для снижения риска ишемических событий [261].
ЕОК IС (УУР С, УДД 5)
- При планируемой ранней инвазивной стратегии лечения (КГ с намерением выполнить реваскуляризацию миокарда в первые 24 ч после госпитализации) у пациентов с ОКСбпST и неизвестной коронарной анатомией рутинное добавление к АСК** ингибитора Р2Y12-рецептора тромбоцитов до получения результатов КГ не рекомендуется из-за возможного увеличения риска неблагоприятных исходов [262-267].
ЕОК IIIA (УУР A, УДД 1)
- Если в первые 24 ч после госпитализации инвазивное лечение (КГ с намерением выполнить реваскуляризацию миокарда) пациента с ОКСбпST не планируется и нет высокого риска кровотечений, рекомендуется добавить к АСК** ингибитор Р2Y12-рецептора тромбоцитов (клопидогрел**, тикагрелор**, прасугрел) с целью улучшения прогноза [228][268].
ЕОК IIаВ (УУР В, УДД 2)
Комментарии. Данная рекомендация касается пациентов с сохраняющимся подозрением на ОКСбпST, когда КГ может быть выполнена в более поздние сроки госпитализации, а также если планируется неинвазивное лечение ОКСбпST.
- Прасугрел (нагрузочная доза 60 мг внутрь, ежедневная поддерживающая доза 10 мг 1 раз/сут. внутрь) в добавление к АСК** сроком на 12 мес. рекомендуется при коронарном стентировании у больных с ОКСбпST, не получавших других ингибиторов Р2Y12-рецепторов тромбоцитов, если к нему нет противопоказаний (внутричерепное кровоизлияние в анамнезе, ишемический инсульт или транзиторная ишемическая атака в анамнезе, продолжающееся кровотечение, тяжелая печеночная недостаточность) для снижения риска ишемических событий (сумма случаев сердечно-сосудистой смерти, ИМ и инсульта; тромбоз стента для КА) [226][262][263][269].
ЕОК IB (УУР В, УДД 2)
Комментарии. Безопасность перехода на прием прасугрела у пациентов, уже получивших клопидогрел**, изучена недостаточно. Лечение праcугрелом не исключает применение блокаторов рецепторов гликопротеина (ГП) IIb/IIIa тромбоцитов при выполнении ЧКВ. У пациентов в возрасте ≥75 лет, с массой тела <60 кг поддерживающую суточную дозу прасугрела необходимо снизить до 5 мг.
- При ОКСбпST прасугрел не рекомендуется использовать до диагностической КГ и принятия решения о ЧКВ из-за увеличения риска кровотечений [3][263].
ЕОК IIIB (УУР В, УДД 2)
- Тикагрелор** (нагрузочная доза 180 мг внутрь, поддерживающая 90 мг внутрь 2 раза/сут.) рекомендуется в добавление к АСК** сроком на 12 мес. у пациентов с ОКСбпST вне зависимости от стратегии лечения и предшествующего использования клопидогрела**, если к нему нет противопоказаний (внутричерепное кровоизлияние в анамнезе, продолжающееся кровотечение) для снижения риска ишемических событий (сумма случаев сердечно-сосудистой смерти, ИМ и инсульта; тромбоз стента для КА) [3][227][270-272].
ЕОК IB (УУР A, УДД 2)
Комментарии. Переход на прием тикагрелора**, начиная с нагрузочной дозы, возможен у пациентов, уже получивших клопидогрел**, в т. ч. в нагрузочной дозе. Лечение тикагрелором** не исключает применение блокаторов рецепторов ГП IIb/IIIa тромбоцитов при выполнении ЧКВ.
- При ранней инвазивной стратегии лечения ОКСбпST (КГ с намерением выполнить ЧКВ с первые часы после госпитализации) в добавление к АСК** рекомендуется предпочесть прасугрел, а не тикагрелор** с целью более оптимального влияния на прогноз [262][273].
ЕОК IIaВ (УУР В, УДД 2)
- У пациентов с ОКСбпST, которые не могут получать тикагрелор** или прасугрел из-за наличия противопоказаний, непереносимости или недоступности, или нуждаются в длительном лечении антикоагулянтами, в добавление к АСК** рекомендуется клопидогрел** (нагрузочная доза 300 или 600 мг внутрь, поддерживающая доза 75 мг внутрь 1 раз/сут.) сроком на 12 мес. для снижения риска неблагоприятных исходов (сумма случаев сердечно-сосудистой смерти, ИМ и инсульта) [3][12][224][225][229].
ЕОК IB (УУР 5, УДД С)
Комментарии. Если выполняется ЧКВ, рекомендуется нагрузочная доза клопидогрела** 600 мг; в остальных случаях — нагрузочная доза 300 мг.
- У пациентов с ОКСбпST пожилого возраста, особенно при наличии высокого риска кровотечений, в добавление к АСК** рекомендуется рассмотреть применение клопидогрела** (а не тикагрелора** или прасугрела) для повышения приверженности к лечению и снижения риска кровотечений [274-276].
ЕОК IIbB (УУР A, УДД 2)
Комментарии. В клинических исследованиях под пожилыми подразумевали пациентов старше 70-80 лет. При принятии решения рекомендуется также учитывать наличие и выраженность старческой астении и сопутствующих заболеваний.
- У пациентов с ОКСбпST, высоким риском последующих ишемических событий и низким риском кровотечений, не переносивших в прошлом инсульт или транзиторную ишемическую атаку, после выполнения процедур реваскуляризации миокарда (если к ним были показания) и прекращения парентерального введения антикоагулянта рекомендуется рассмотреть добавление к сочетанию АСК** и клопидогрела** ривароксабан** в дозе 2,5 мг 2 раза/сут. внутрь сроком на 12 мес. (в отдельных случаях — вплоть до 24 мес.) для снижения риска неблагоприятных исходов (сумма случаев сердечно-сосудистой смерти, ИМ и инсульта; тромбоз стентов для КА***) [230][277].
ЕОК IIbB (УУР B, УДД 2)
Комментарии. Прием ривароксабана** в дозе 2,5 мг 2 раза/сут. в добавление к сочетанию АСК** и клопидогрела** рекомендуется начинать в первую неделю после начала лечения ОКСбпST [230].
Добавление ривароксабана** в дозе 2,5 мг 2 раза/сут. не изучено в сочетании с двойной антитромбоцитарной терапией, в состав которой входят прасугрел или тикагрелор**. Данный подход не может использоваться у пациентов, нуждающихся в использовании более высоких доз антикоагулянтов (в частности, при наличии ФП).
- В/в введение ингибиторов ГП IIb/IIIa рецептора тромбоцитов (АТХ-группа Антиагреганты, кроме гепарина, B01AC) (абциксимаб, тирофибан, эптифибатид, фрагменты моноклональных антител FRaMon F[ab']2) рекомендуется только как спасительное средство при возникновении тромботических осложнений или феномена slow flow/no-reflow во время ЧКВ [3][278][279].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. Ингибиторы ГП IIb/IIIa тромбоцитов были изучены преимущественно до начала широкого применения ингибиторов Р2Y12-рецептора тромбоцитов [278][279]. Помимо осложнений во время ЧКВ введение ингибитора ГП IIb/IIIa может рассматриваться при ЧКВ высокого риска у больных, не получивших ингибитор Р2Y12-рецептора тромбоцитов, а также наличии массивного интракоронарного тромбоза.
- У пациентов с ОКСбпST и высоким риском желудочно-кишечных кровотечений во время двойной антитромбоцитарной терапии для защиты слизистой оболочки желудка и снижения риска желудочно-кишечных кровотечений рекомендуется использовать ингибиторы протонного насоса [280-284].
ЕОК IA (УУР A, УДД 2)
Комментарии. Риск желудочно-кишечных кровотечений повышен при язвенной болезни или желудочно-кишечном кровотечении в анамнезе, хроническом использование нестероидных противовоспалительных средств или кортикостероидов, а также при наличии как минимум 2 из следующих признаков — возраст ≥65 лет, диспепсия, желудочно-пищеводный рефлюкс, инфицирование Helicobacter pylori, хроническое употребление алкоголя.
Известно о возможном ослаблении антитромбоцитарного эффекта клопидогрела** при его сочетании с омепразолом** или эзомепразолом**, но не с пантопразолом или рабепразолом, при лабораторной оценке активности тромбоцитов. Нет доказательств, что эти лекарственные взаимодействия оказывают неблагоприятное влияние на клинические результаты лечения [282-286].
- В связи с высоким риском кровотечений, превышающим потенциальную пользу, у пациентов с ОКСбпST не рекомендуется применять ACК** при уровне тромбоцитов <10×10⁹/л, клопидогрел** — <30×10⁹/л, тикагрелор** или прасугрел — <50×10⁹/л [3][287].
ЕОК IIIС (УУР C, УДД 5)
Альтернативные подходы к выбору длительности и состава двойной антитромбоцитарной терапии (сочетания АСК** с ингибитором Р2Y12-рецептора тромбоцитов) в первые 12 мес. после ОКСбпST
В определении длительности двойной антитромбоцитарной терапии у пациентов, перенесших ОКСбпST, большую роль играет оценка риска ишемических и геморрагических осложнений.
- У пациентов с ОКСбпST, подвергнутых ЧКВ, без высокого риска ишемических осложнений, не имевших ишемических осложнений в период двойной антитромбоцитарной терапии (сочетание АСК** с ингибитором Р2Y12-рецептора тромбоцитов), рекомендуется ограничить ее продолжительность 3-6 мес., перейдя на монотерапию ингибитором Р2Y12-рецептора тромбоцитов, для снижения риска кровотечений и опосредованного снижения риска ишемических событий [288-294].
ЕОК IIaA (УУР A, УДД 2)
Комментарии. В настоящее время основным подходом является 12-мес. длительность двойной антитромбоцитарной терапии. "Ранний" переход с двойной антитромботической терапии на монотерапию антиагрегантом после ЧКВ наиболее освоен при отмене АСК** с переходом на монотерапию тикагрелором** в дозе 90 мг 2 раза/сут.
- У пациентов с ОКСбпST и высоким риском кровотечений рекомендуется рассмотреть переход с сочетания АСК** с ингибитором Р2Y12-рецептора тромбоцитов на монотерапию ингибитором Р2Y12-рецептора тромбоцитов или АСК** через 1 мес. после начала лечения [295][296].
ЕОК IIbB (УУР B, УДД 2)
Комментарии. Столь "ранний" переход с двойной антитромбоцитарной терапии на монотерапию антиагрегантом выглядит наиболее безопасным с точки зрения ишемических осложнений при отмене АСК** с переходом на монотерапию тикагрелором** в дозе 90 мг 2 раза/сут. у пациентов, подвергнутых ЧКВ; переход на монотерапию клопидогрелом** у пациентов с ОКСбпST, подвергнутых ЧКВ, в исследовании STOPDAPT-2 ACS был сопряжен с увеличением риска ИМ [296].
- У пациентов с ОКСбпST, подвергнутых ЧКВ, не имевших ишемических осложнений в первый месяц двойной антитромбоцитарной терапии, рекомендуется рассмотреть переход с сочетания АСК** с тикагрелором** или прасугрелом на сочетание АСК** с клопидогрелом** ("деэскалация" двойной антитромбоцитарной терапии) для снижения риска кровотечений и опосредованного снижения риска ишемических событий [297-300].
ЕОК IIbA (УУР В, УДД 2)
Комментарии. К настоящему времени "деэскалация" двойной антитромбоцитарной терапии с заменой прасугрела или тикагрелора** на клопидогрел** в первый месяц после начала лечения ОКС не рекомендуется. Подход к "деэскалации" двойной антитромбоцитарной терапии на основании лабораторных данных (с учетом остаточной реактивности тромбоцитов, полиморфизмов изофермента цитохрома Р450 С19), изученный при ОКСбпST в более ранние сроки после ЧКВ, не имеет преимуществ перед деэскалацией без учета лабораторных показателей.
Возможные основания для перехода с прасугрела или тикагрелора** на клопидогрел** в первые 12 мес. после ОКСбпST: появление или повторение клинически значимых кровотечений, причину которых невозможно выявить или устранить; небольшие повторяющиеся ("надоедливые") кровотечения, источник которых устранить не удается, сказывающиеся на приверженности к лечению; побочные эффекты тикагрелора** (одышка, клинические проявления гиперурикемии); возникновение показаний к длительному лечению антикоагулянтами; возникновение ишемического инсульта или транзиторная ишемическая атака у получающих прасугрел; желание пациента уменьшить кратность приема препарата (переход с тикагрелора** на клопидогрел**) и число принимаемых таблеток (переход с тикагрелора** или прасугрела на фиксированную комбинацию АСК** с клопидогрелом**) с целью улучшения приверженности к лечению; ограниченная доступность прасугрела или тикагрелора**, не позволяющая обеспечить их регулярный прием.
Спустя 30 дней от начала лечения ОКС рекомендуются следующие способы перехода с тикагрелора** или прасугрела на клопидогрел**: прием внутрь 75 мг клопидогрела** через 24 ч от последнего приема прасугрела; прием внутрь 600 мг клопидогрела** через 24 ч от последнего приема тикагрелора**. При переходе на клопидогрел** из-за кровотечения или при опасности кровотечения рекомендуется рассмотреть начало приема клопидогрела** с поддерживающей дозы (75 мг). В последующем доза клопидогрела** — 75 мг 1 раз/сут.
3.2.3.2. Парентеральные антикоагулянты
- У пациентов с подтвержденным диагнозом ОКСбпST рекомендуется парентеральное введение антикоагулянтов (группа B01А — антитромботические средства), если к ним нет противопоказаний, с целью снижения риска неблагоприятного исхода (сумма случаев смерти и ИМ). При выборе препарата следует учитывать риск ишемических осложнений и кровотечений, стратегию лечения пациента, эффективности и безопасности конкретного антикоагулянта [231][301].
ЕОК IA (УУР A, УДД 1)
Комментарии. Антикоагулянты должны использоваться в сочетании с антиагрегантами.
У пациентов, уже получающих (до развития ОКСбпST) антагонисты витамина К, рекомендуется не использовать парентеральное введение антикоагулянтов (в т. ч. во время ЧКВ) при условии, что поддерживаются значения МНО ≥2,0 (при ЧКВ ≥2,5).
У пациентов, получающих прямые пероральные антикоагулянты, рекомендуется использование в/в болюса гепарина натрия** (син.: нефракционированный гепарин (НФГ)) во время ЧКВ независимо от времени, прошедшего после приема последней дозы прямого перорального антикоагулянта; аналогичный подход рекомендуется у пациентов, получающих антагонисты витамина К, при МНО <2,5.
- После успешного ЧКВ у пациентов с ОКСбпST рекомендуется прекратить парентеральное введение антикоагулянта из-за опасности увеличения риска кровотечений [3].
ЕОК IIaC (УУР C, УДД 5)
Комментарии. У пациентов с показаниями к длительному применению антикоагулянтов (ФП/ТП, механический протез клапанов сердца, тромбоз полости ЛЖ, профилактики или лечение венозного тромбоза, ТЭЛА) рекомендуется продолжить введение парентерального антикоагулянта в соответствующих дозах или рассмотреть переход на пероральные антикоагулянты. Если реваскуляризация миокарда не проводилась и нет показаний к длительному применению антикоагулянтов, лечение парентеральным антикоагулянтом должно продолжаться вплоть до 8-х сут. (с более ранним прекращением, если в эти сроки было выполнено ЧКВ или больной был выписан); минимальная длительность парентерального введения антикоагулянта при неинвазивном лечении ОКСбпST составляет 2 сут.
- При выполнении ЧКВ в первые 24 ч от начала лечения ОКСбпST для снижения риска ишемических событий рекомендуется в/в болюсное введение НФГ** в дозе 70-100 МЕ/кг [3].
ЕОК IС (УУР С, УДД 5)
Комментарии. При начале лечения до ЧКВ рекомендуется в/в инфузия НФГ** в дозе, обеспечивающей АЧТВ в 1,5-2,5 раза выше контрольного для данной лаборатории.
Во время ЧКВ НФГ** рекомендуется ввести в/в болюсом 70-100 МЕ/кг; необходимость повторных болюсов определяется величиной активированного времени свертывания крови (АВС). Аналогичные подходы рекомендуется использовать при срочном ЧКВ у больных, получающих прямые пероральные антикоагулянты. Если ЧКВ выполняется на фоне начатой в/в инфузии НФГ**, во время процедуры рекомендуется рассмотреть дополнительное в/в введение болюсов НФГ** под контролем АВС.
- При выполнении КГ в первые 24 ч от начала лечения ОКСбпST в качестве альтернативы НФГ** для снижения риска ишемических событий рекомендуется эноксапарин натрия** [234][302-304].
ЕОК IIaB (УУР A, УДД 2)
Комментарии. Эноксапарин натрия** не рекомендуется при рСКФ <15 мл/мин/1,73 м². Переход с НФГ** на эноксапарин натрия** и с эноксапарина натрия** на НФГ** не рекомендуется из-за увеличения риска кровотечений.
- Если пациент с ОКСбпST получал эноксапарин натрия** до ЧКВ, его следует продолжить и во время процедуры; при этом если после подкожной инъекции до раздувания баллона в КА прошло >8 ч, рекомендуется дополнительный в/в болюс препарата 0,3 мг/кг [234][302][305][306].
ЕОК IIaB (УУР A, УДД 2)
- Если в ранние сроки лечения ОКСбпST не планируется выполнение КГ, для снижения риска ишемических событий рекомендуется фондапаринукс натрия (2,5 мг подкожно ежедневно) как имеющий наиболее благоприятный профиль эффективности и безопасности, если к нему нет противопоказаний [232][233][236].
ЕОК IB (УУР A, УДД 2)
Комментарии. Больному, получающему фондапаринукс натрия, во время ЧКВ следует в/в вводить НФГ** — первоначальный болюс в дозе 85 МЕ/кг; необходимость повторных болюсов определяется величиной АВС крови. Фондапаринукс натрия противопоказан при рСКФ <20 мл/мин/1,73 м².
Если фондапаринукс натрия недоступен, может использоваться эноксапарин натрия**.
- В случаях, когда пациенту с ОКСбпST противопоказаны фондапаринукс натрия и эноксапарин натрия** (например, из-за выраженного нарушения фильтрационной функции почек), для снижения риска ишемических событий рекомендуется НФГ** [3].
ЕОК IB (УУР C, УДД 5)
3.2.3.3. Антитромботическая терапия при наличии показаний к длительному пероральному приему антикоагулянтов
- У пациентов с ОКСбпST и показаниями к длительному применению антикоагулянтов, которые на момент начала ОКСбпST их не принимали, рекомендуется начинать антитромботическое лечение с тройной антитромботической терапии (сочетание АСК**, ингибитора Р2Y12-рецептора тромбоцитов и парентерального антикоагулянта) с последующей заменой парентерального антикоагулянта на пероральный (апиксабан**, дабигатрана этексилат**, ривароксабан**, эдоксабан или антагонист витамина K) [3][218-252][307].
ЕОК IA (УУР 5, УДД С)
Комментарии. Показания к длительному применению антикоагулянтов — ФП/ТП, механический протез клапанов сердца, тромбоз полости ЛЖ, тромбоз глубоких вен и/или ТЭЛА.
У пациентов, уже получающих (до развития ИМпST) антагонисты витамина К, рекомендуется не использовать парентеральное введение антикоагулянтов (в т. ч. во время ЧКВ) при условии, что поддерживаются значения МНО ≥2,0 (при ЧКВ ≥2,5).
У пациентов, получающих прямые пероральные антикоагулянты, рекомендуется использование в/в болюса НФГ** во время ЧКВ независимо от времени, прошедшего после приема последней дозы прямого перорального антикоагулянта; аналогичный подход рекомендуется у пациентов, получающих антагонисты витамина К, при значениях МНО <2,5.
- У пациентов с ОКСбпST и показаниями к длительному применению антикоагулянтов, подвергнутых стентированию КА, длительность тройной антитромботической терапии (сочетание антикоагулянта с АСК** и клопидогрелом**) рекомендуется ограничить несколькими днями (1-7 дней) для уменьшения риска геморрагических осложнений. Через 1-7 дней, после отмены АСК**, рекомендуется перейти на двойную антитромботическую терапию (пероральный антикоагулянт в сочетании с клопидогрелом**) [216][246-248][308].
ЕОК IA (УУР A, УДД 2)
Комментарии. В составе двойной антитромботической терапии следует предпочесть клопидогрел**, а не АСК**. У отдельных пациентов с очень высоким риском тромбоза КА и низким риском кровотечений может быть рассмотрено применение не клопидогрела**, а тикагрелора** в течение 6 мес.
- У пациентов с ОКСбпST и показаниями к длительному применению антикоагулянтов, подвергнутых стентированию КА и имеющих высокий риск коронарных осложнений (включая особенности коронарной анатомии и/или инвазивного вмешательства), у которых ожидаемая польза представляется выше риска кровотечений, для снижения риска тромбоза стента рекомендуется продление тройной антитромботической терапии (сочетания перорального антикоагулянта с АСК** и клопидогрелом**) до 1 мес. с последующей отменой АСК** и переходом на двойную антитромботическую терапию (сочетание перорального антикоагулянта с клопидогрелом**) [3][247].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. У отдельных пациентов с очень высоким риском тромбоза КА и низким риском кровотечений может быть рассмотрено применение не клопидогрела**, а тикагрелора** в течение 6 мес.
- У пациентов с ОКСбпST, нуждающихся в длительном применении антикоагулянтов, которым не выполнялось стентирование КА, рекомендуется прием перорального антикоагулянта в сочетании с одним антиагрегантом для снижения риска кровотечений [247].
ЕОК IIaB (УУР B, УДД 2)
Комментарии. Продленная тройная антитромботическая терапия (сочетание антикоагулянта в двумя антиагрегантами более, чем в течение 1 нед.) у пациентов, которым не выполнялось стентирование КА, не рекомендуется из-за высокой частоты кровотечений, но может быть рассмотрена в индивидуальном порядке у пациентов с низким риском кровотечений и очень высоким риском коронарных осложнений. В качестве антиагрегантов в сочетании с пероральным антикоагулянтом у больных, которым не выполнялось стентирование КА, рекомендуется использовать клопидогрел** или АСК**.
- У пациентов с ОКСбпST и сохраняющимися показаниями к применению антикоагулянтов двойная антитромботическая терапия (пероральный антикоагулянт в сочетании с клопидогрелом**, реже — с АСК**) рекомендуется вплоть до 12 мес., после чего клопидогрел** (или АСК**) рекомендуется отменить, продолжив монотерапию пероральным антикоагулянтом в дозе, необходимой для профилактики и/или лечения тромботических и/или тромбоэмболических осложнений [218-252][307].
ЕОК IA (УУР A, УДД 2)
Комментарии. У пациентов с высоким риском кровотечений возможен переход на монотерапию пероральным антикоагулянтом через 6 мес. после ЧКВ. Аналогичный подход можно рассматривать и у больных с ОКСбпST, нуждающихся в длительном пероральном приеме антикоагулянтов.
У отдельных пациентов с низким риском кровотечений и сохраняющимся высоким риском коронарных осложнений может быть рассмотрено продление двойной антитромботической терапии (сочетания перорального антикоагулянта с клопидогрелом** или АСК**) более чем на 12 мес.
- У пациентов с ОКСбпST и неклапанной ФП, нуждающихся в сочетании антикоагулянтов и антиагрегантов, рекомендуется предпочесть прямые пероральные антикоагулянты, если к ним нет противопоказаний, а не антагонисты витамина К [3].
ЕОК IB (УУР C, УДД 5)
Комментарии. Данные о сравнительной эффективности прямых пероральных антикоагулянтов и варфарина** у пациентов с ОКС ограничены анализом подгрупп в рандомизированных контролируемых исследованиях.
- У пациентов с ОКСбпST и неклапанной ФП, нуждающихся в сочетании антикоагулянтов и антиагрегантов, для предотвращения тромбоэмболических осложнений рекомендуется апиксабан** в дозе 5 мг 2 раза/сут. или 2,5 мг 2 раза/сут., если к нему нет противопоказаний [252].
ЕОК IIaB (УУР B, УДД 2)
Комментарии. По имеющимся данным, апиксабан** безопаснее варфарина** в составе двойной и тройной антитромботической терапии при сходной с варфарином** частоте коронарных осложнений. Апиксабан** изучен как на больных с ОКС, подвергнутых стентированию КА, так и при неинвазивном лечении ОКС.
Стандартная доза апиксабана** в этой клинической ситуации — 5 мг 2 раза/сут. При наличии как минимум 2 из указанных факторов — возраст ≥80 лет, масса тела ≤60 кг, креатинин в крови ≥133 мкмоль/л — используется доза 2,5 мг 2 раза/сут.
- У пациентов с ОКСбпST и неклапанной ФП, нуждающихся в сочетании антикоагулянтов и антиагрегантов, для предотвращения тромбоэмболических осложнений рекомендуется дабигатрана этексилат** в дозе 150 мг 2 раза/сут. или 110 мг 2 раза/сут., если к нему нет противопоказаний [246][251].
ЕОК IIaB (УУР B, УДД 2)
Комментарии. Дабигатрана этексилат** в дозах 110 или 150 мг 2 раза/сут. в составе двойной антитромботической терапии (в сочетании преимущественно с клопидогрелом**) у пациентов, подвергнутых коронарному стентированию, безопаснее тройной антитромботической терапии (сочетание варфарина**, преимущественно клопидогрела** и АСК**). При этом отмечена тенденция к большей частоте коронарных осложнений на дозе дабигатрана этексилата** 110 мг 2 раза/сут. Поэтому в составе двойной антитромботической терапии (в комбинации с клопидогрелом**) после коронарного стентирования рекомендуется использовать дабигатрана этексилат** в дозе 150 мг 2 раза/сут.; дозу 110 мг 2 раза/сут. в составе двойной антитромботической терапии можно предпочесть у больных с преобладающим риском кровотечений. В составе тройной антитромботической терапии при неклапанной ФП предпочтительнее доза 110 мг 2 раза/сут.
- У пациентов с ОКСбпST и неклапанной ФП, нуждающихся в сочетании антикоагулянтов и антиагрегантов, для предотвращения тромбоэмболических осложнений рекомендуется ривароксабан** в дозе 20 мг 1 раз/сут. (при клиренсе креатинина 30-49 мл/мин — 15 мг 1 раз/сут.) или 15 мг 1 раз/сут. (при клиренсе креатинина 30-49 мл/мин — #10 мг 1 раз/сут.) при преобладающем риске кровотечений, если к нему нет противопоказаний [3][245][309].
ЕОК IB (УУР С, УДД 5)
Комментарии. Ривароксабан** в дозе 15 мг 1 раз/сут. (при клиренсе креатинина 30-49 мл/мин — #10 мг 1 раз/сут.) в сочетании с преимущественно клопидогрелом** у больных, подвергнутых стентированию КА, безопаснее тройной антитромботической терапии (сочетание варфарина**, преимущественно клопидогрел** и АСК**). Если риск кардиоэмболических и коронарных осложнений превышает опасность кровотечений, рекомендуется применение стандартных доз ривароксабана**, рекомендованных для больных с неклапанной ФП (20 мг 1 раз/сут., при клиренсе креатинина 30-49 мл/мин — 15 мг 1 раз/сут.).
- У пациентов с ОКСбпST и неклапанной ФП при применении антагонистов витамина К в сочетании с антитромбоцитарными препаратами для снижения риска кровотечений рекомендуется поддерживать МНО в диапазоне 2,0-2,5, а время нахождения МНО в терапевтическом диапазоне — не <70% [241][245][249-252][307][310].
ЕОК IIaB (УУР С, УДД 5)
Комментарии. Целевые значения МНО при других показаниях к антагонисту витамина К могут быть выше (например, у больных с механическими протезами клапанов сердца, при тромбоэмболических осложнениях на фоне указанных значений МНО).
- Для снижения риска кровотечений во время ЧКВ у пациентов, получающих пероральные антикоагулянты, рекомендуется использовать лучевой доступ [165][166].
ЕОК IIaA (УУР А, УДД 1)
- При раннем инвазивном лечении ОКСбпST у пациентов, получающих пероральный антикоагулянт, для уменьшения риска кровотечений не рекомендуется использовать ингибиторы Р2Y12-рецептора тромбоцитов до получения результатов КГ и принятия решения о выполнении ЧКВ, а целесообразность применения ингибиторов Р2Y12-рецептора тромбоцитов рассматривать только при очевидных тромботических осложнениях ЧКВ [3][278][279].
ЕОК IIIC (УУР С, УДД 5)
- Для защиты слизистой оболочки желудка и снижения риска желудочно-кишечных кровотечений во время применения тройной антитромботической терапии (сочетание перорального антикоагулянта с ингибитором Р2Y12-рецептора тромбоцитов и АСК**) или двойной антитромботической терапии (сочетание перорального антикоагулянта с ингибитором Р2Y12-рецептора тромбоцитов или АСК**) у пациентов с высоким риском желудочно-кишечных кровотечений рекомендуется использовать ингибиторы протонного насоса [311-314].
РКО IA (УУР В, УДД 2)
Комментарии. Риск желудочно-кишечных кровотечений повышен при язвенной болезни или желудочно-кишечном кровотечении в анамнезе, хроническом использовании нестероидных противовоспалительных средств или кортикостероидов, а также при наличии как минимум 2 из следующих признаков — возраст ≥65 лет, диспепсия, желудочно-пищеводный рефлюкс, инфицирование Helicobacter pylori, хроническое употребление алкоголя.
Известно о возможном ослаблении антитромбоцитарного эффекта клопидогрела** при его сочетании с омепразолом** или эзомепразолом**, но не с пантопразолом или рабепразолом, при лабораторной оценке активности тромбоцитов. Нет доказательств, что эти лекарственные взаимодействия оказывают неблагоприятное влияние на клинические результаты лечения [280-286].
3.2.3.4. Антитромботическая терапия через 12 мес. после ОКСбпST у пациентов, не имеющих показаний к длительному пероральному приему антикоагулянтов
- У пациентов, перенесших ОКСбпST, с высоким риском коронарных осложнений и низким риском кровотечений, не имевших кровотечений в течение первого года лечения, для снижения риска смерти, повторного ИМ, суммарного риска ишемических событий рекомендуется продление двойной антитромботической терапии (сочетание АСК** с ингибитором Р2Y12-рецепторов тромбоцитов или ривароксабаном** в дозе 2,5 мг 2 раза/сут.) на более длительный срок. При этом в ходе наблюдения соотношение пользы и риска продления двойной антитромботической терапии должно регулярно оцениваться повторно [3][315-322].
ЕОК IIaА (УУР B, УДД 2)
Комментарии. В пользу высокого риска коронарных осложнений свидетельствует сочетание коронарной болезни сердца в сочетании с одним из следующих критериев: СД, требующий лечения; повторные ИМ в анамнезе; многососудистая коронарная болезнь сердца; сочетание коронарного и периферического атеросклероза; преждевременная коронарная болезнь сердца (в возрасте до 45 лет); ускоренное развитие коронарной болезни сердца (два острых коронарных события за предшествующие 2 года), хроническое системное воспалительное заболевание (системная красная волчанка, артрит, носxительство вируса иммунодефицита человека); рСКФ 15-59 мл/мин/1,73 м²; технические аспекты выполненного ЧКВ — имплантация как минимум 3 стентов, вмешательство как минимум на 3 стенозах, общая длина стентов >60 мм, комплексная реваскуляризация (стентирование ствола левой КА, бифуркационное стентирование как минимум 2 стентами, стентирование хронических окклюзий, стентирование последнего проходимого сосуда); тромбоз стента в анамнезе на фоне лечения антиагрегантами.
О высоком риске кровотечений свидетельствуют внутричерепное кровотечение, ишемический инсульт или другая внутричерепная патология в анамнезе, недавнее желудочно-кишечное кровотечение или анемия из-за потери крови через желудочно-кишечный тракт, другая патология желудочно-кишечного тракта с повышенным риском кровотечений, печеночная недостаточность, геморрагический диатез или коагулопатия, старческий возраст или старческая астения, ХБП, требующая диализа, или с рСКФ <15 мл/мин/1,73 м².
- У пациентов, перенесших ОКСбпST, с высоким риском коронарных осложнений, не имевших кровотечений в первый год двойной антитромбоцитарной терапии, для снижения риска ишемических событий рекомендуется ее продление в виде сочетания АСК** с уменьшенной дозой тикагрелора** (60 мг 2 раза/сут. внутрь) [320][321].
ЕОК IIаB (УУР B, УДД 2)
Комментарии. В исследовании PEGASUS-TIMI 54, продемонстрировавшем целесообразность такого подхода, к пациентам, имеющим высокий риск коронарных осложнений, относили лиц ≥50 лет, перенесших ИМ, в сочетании как минимум с одним из следующих факторов риска: возраст ≥65 лет, наличие требующего медикаментозного лечения СД, ≥2 перенесенных ИМ, многососудистый коронарный атеросклероз, ХБП с рСКФ <60 мл/мин/1,73 м². Изученная длительность применения сочетания АСК** с тикагрелором** в дозе 60 мг 2 раза/сут. составляет 36 мес.
- У пациентов, перенесших ОКСбпST, с высоким риском тромботических осложнений атеросклероза, не имевших кровотечений в течение первого года лечения, для снижения риска ишемических событий рекомендуется длительное использование сочетания АСК** с ривароксабаном** в дозе 2,5 мг 2 раза/сут. внутрь [322].
ЕОК IIаB (УУР B, УДД 2)
Комментарии. В исследовании COMPASS [202], продемонстрировавшем целесообразность такого подхода, к пациентам с высоким риском тромботических осложнений атеросклероза относили лиц, перенесших ИМ или имеющих многососудистый коронарный атеросклероз, в случаях: возраст ≥65 лет, наличие атеросклеротического поражения других (не коронарных) сосудистых бассейнов, или как минимум 2 из следующих факторов риска: курение, СД, нетяжелая ХСН в анамнезе, нелакунарный ишемический инсульт в анамнезе, ХБП с рСКФ <60 мл/мин/1,73 м².
- У пациентов, перенесших ОКСбпST, через 12 мес. рекомендуется рассмотреть переход не на монотерапию АСК**, а на монотерапию ингибитором Р2Y12-рецепторов тромбоцитов для дополнительного снижения риска ишемических событий [323][324].
ЕОК IIbA (УУР B, УДД 2)
Комментарии. Дополнительная польза монотерапии клопидогрелом** наиболее очевидна у пациентов, 1 год назад подвергнутых коронарному стентированию (рандомизированное контролируемое исследование HOST-EXAM) [324]. Опубликованы также данные в пользу монотерапии #тикагрелором** в дозе 90 мг 2 раза/сут. (анализ GLASSY исследования GLOBAL LEADERS) [325][326].
3.2.4. Органические нитраты
- Из-за отсутствия доказательств положительного влияния на прогноз рутинное назначение органических нитратов в виде в/в инфузии, трансдермально или перорально при ОКСбпST не рекомендуется [327][328][373].
ЕОК IIIB (УУР А, УДД 2)
Комментарии. При ОКСбпST нитраты назначают только по показаниям, к которым прежде всего относят постинфарктную стенокардию. Иногда нитраты назначают по поводу СН, хотя для этой цели лучше подходят ингибиторы ангиотензинпревращающего фермента (иАПФ). Для профилактики толерантности к нитратам их назначают прерывисто: промежуток времени между последним приемом препарата и первым приемом на следующий день должен быть не менее 12 ч (оптимально — 16 ч).
- В/в инфузия нитратов (нитроглицерина** или изосорбида динитрата**) рекомендуется для симптоматического лечения у пациентов с ОКСбпST и продолжающейся ишемией миокарда, АГ, СН при отсутствии противопоказаний [329-332].
ЕОК IC (УУР B, УДД 2)
Комментарии. Критерий адекватно подобранной скорости введения (дозы) при в/в инфузии нитратов — уровень систолического АД, который должен быть снижен на 10-15% у нормотоников и на 25-30% — у лиц с АГ, но не ниже 100 мм рт.ст. Обычная начальная скорость введения нитроглицерина** 10 мкг/мин. При ее неэффективности скорость инфузии увеличивается на 10-15 мкг/мин каждые 5-10 мин, пока не будет достигнут желаемый эффект. Если достичь целевого уровня снижения АД не удается, даже увеличив скорость инфузии нитроглицерина до 166 мкг/мин, то дальнейшее увеличение дозы не имеет смысла. Оптимальная продолжительность инфузии нитратов — не более 24-48 ч, т. к. в дальнейшем часто развивается толерантность. При развитии гипотонии обычно достаточно прекратить инфузию нитроглицерина**, т. к. у препарата короткий период полувыведения. Реже приходится проводить стандартные мероприятия по увеличению притока крови к сердцу (приподнять нижние конечности; возможно в/в введение 0,9% раствора натрия хлорида**, применение адрено- и допаминстимуляторов).
Органические нитраты не рекомендуются при артериальной гипотонии, ИМ ПЖ, а также после приема силденафила или варденафила в предыдущие 24 ч, тадалафила — в предыдущие 48 ч из-за высокого риска осложнений. При появлении головной боли могут использоваться анальгетики и кофеинсодержащие препараты.
- У пациентов с ОКСбпST рекомендуется применение органических нитратов при подозрении на вазоспастический механизм развития ОКС, а также при доказанной вазоспастической стенокардии — для купирования и профилактики приступов стенокардии [327][328][330].
ЕОК IIaB (УУР B, УДД 2)
3.2.5. Бета-адреноблокаторы
- Пациентам с ОКСбпST, с АГ и/или сохраняющейся ишемией миокарда и/или тахикардией, не имеющим признаков ОСН, для контроля за ишемией и профилактики ЖА рекомендуется в/в введение бета-адреноблокатора (β-АБ) (преимущественно метопролола**; Приложение А3. Медикаментозное лечение ОКСбпST; Приложение А3. Дозы антитромботических лекарственных средств при нарушенной функции почек) при отсутствии противопоказаний [333-339].
ЕОК IIaB (УУР A, УДД 1)
Комментарии. Пациентам с ОКСбпST при отсутствии признаков ОСН рекомендуется в/в введение первоначальной дозы β-АБ, особенно при наличии АГ и/или сохраняющейся ишемии миокарда и/или тахикардии, с последующим переходом на прием препаратов внутрь. В/в β-АБ предотвращают развитие жизнеугрожающих ЖА [340].
Есть данные, что польза от β-АБ тем выше, чем раньше начата терапия и чем быстрее проявляется их действие.
- При ОКСбпST длительный пероральный прием β-АБ рекомендуется у пациентов с ФВ ЛЖ ≤40% вне зависимости от наличия СН для снижения риска смерти, при отсутствии противопоказаний [92][333][335][341-345].
ЕОК IA (УУР А, УДД 1)
Комментарии. У данной категории пациентов рекомендуется продолжить или начать применение одного из трех β-АБ c доказанным положительным влиянием на смертность при ХСН с низкой ФВ ЛЖ (метопролола** с замедленным высвобождением действующего вещества, бисопролола** (группа C07AB — селективные β-АБ) или карведилола** (группа C07AG — альфа- и β-АБ)) или при ИМ с СН с низкой ФВ ЛЖ (карведилол**) и при хорошей переносимости стремиться достичь целевых доз, обеспечивающих благоприятное влияние на прогноз (Приложение А3. Медикаментозное лечение ОКСбпST; Приложение А3. Дозы антитромботических лекарственных средств при нарушенной функции почек).
Данные о целесообразности применения β-АБ для улучшения прогноза после ОКСбпST у пациентов с ФВ ЛЖ >40% неоднозначны. В настоящее время проводится ряд рандомизированных исследований для оценки пользы назначения β-АБ пациентам с ФВ ЛЖ >40% (BETAMI, DANBLOCK, REBOOT) и ФВ ЛЖ >50% (REDUCE-AMI) [346-349]; их результаты могут повлиять на рекомендации.
Вместе с тем результаты некоторых регистров, ретроспективных исследований и метаанализов говорят о том, что назначение β-АБ приводит к улучшению прогноза [350-353], однако не все регистры говорят об однозначной пользе назначения β-АБ пациентам с сохранной ФВ ЛЖ без СН [354-356]. В целом в настоящее время представляется, что польза не исключена у всех пациентов, перенесших ИМбпST, по крайней мере в течение ближайшего 1 года.
Гемодинамически стабильным пациентам с ОКСбпST β-АБ могут быть назначены в первые 24 ч после начала болезни. В ранние сроки ОКСбпST важнейшее значение имеет выбор приемлемой дозы препарата, которая не должна быть слишком большой при опасности возникновения осложнений (прежде всего — СН). О достаточности дозы обычно судят по достигнутой ЧСС. Она не должна быть ниже 44-46 уд./мин в ночные часы в покое.
Абсолютные противопоказания к использованию β-АБ, в т. ч. при ОКСбпST: кардиогенный шок, тяжелая обструктивная болезнь легких в стадии обострения, атриовентрикулярная (АВ) блокада II-III степени у пациентов без функционирующего искусственного водителя ритма сердца, аллергия (непереносимость компонентов лекарственного препарата).
Относительные противопоказания к использованию β-АБ: клинические проявления СН, свидетельства наличия низкого сердечного выброса, систолическое АД <100 мм рт.ст., ЧСС <60 уд./мин, удлинение интервала PQ >0,24 сек, обструктивная болезнь легких в анамнезе, наличие факторов риска возникновения кардиогенного шока.
У пациентов с существенным нарушением сократимости ЛЖ начинать лечение следует с минимальных доз β-АБ. Через 24-48 ч после исчезновения выраженной брадикардии, артериальной гипотензии, тяжелой СН, АВ-блокады можно начать аккуратное титрование дозы препаратов для приема внутрь. При наличии исходных противопоказаний к β-АБ возможность их назначения следует регулярно пересматривать. Следует воздержаться от назначения β-АБ с внутренней симпатомиметической активностью.
3.2.6. Блокаторы кальциевых каналов
- Из-за отсутствия доказательств эффективности у пациентов с ОКСбпST не рекомендуется рутинное назначение блокаторов кальциевых каналов [357-365].
ЕОК IIIA (УУР A, УДД 1)
Комментарии. В связи с возможностью неконтролируемой гипотонии у пациентов с ОКСбпST следует избегать назначения нифедипина** (группа C08CA — производные дигидропиридина) короткого действия [358-360].
- У пациентов с ОКСбпST, возобновляющейся ишемией миокарда и противопоказаниями к β-АБ для устранения симптомов рекомендуются верапамил** (при наличии АГ может быть использован дилтиазем), если нет клинически значимой сократительной дисфункции ЛЖ, повышенного риска кардиогенного шока, продолжительности интервала PQ >0,24 с, АВ-блокад 2-й или 3-й степени без установленного ЭКС [357][361-365].
ЕОК IIbB (УУР B, УДД 2)
Комментарии. Верапамил**, дилтиазем (группа C08DA — производные фенилалкиламина) или амлодипин** (группа C08CA — производные дигидропиридина) могут применяться при невозможности контролировать АГ другими средствами.
Дилтиазем или верапамил** могут быть рассмотрены для контроля сердечного ритма при невозможности использовать β-АБ, реже — для купирования суправентрикулярных тахиаритмий.
У пациентов с сохраняющейся ишемией миокарда при недостаточной эффективности β-АБ, противопоказаниях к β-АБ или их неприемлемых осложнениях можно рассмотреть добавление длительно действующих дигидропиридиновых производных.
Совместный прием верапамила** и дилтиазема с β-АБ в целом нежелателен из-за суммирования рисков побочных эффектов.
- При подозрении на вазоспастический генез ОКСбпST или при доказанной вазоспастической стенокардии рекомендуются верапамил**, дилтиазем или длительно действующие дигидропиридиновые производные для устранения симптомов и профилактики ишемии миокарда [366].
ЕОК IIaB (УУР С, УДД 5)
3.2.7. Блокаторы ренин-ангиотензин-альдостероновой системы
иАПФ и антагонисты рецепторов ангиотензина II (АРА)
- У пациентов с ОКСбпST при ФВ ЛЖ <40%, АГ, СД, ХБП рекомендуются иАПФ для предотвращения дисфункции ЛЖ, СН и смерти, если к препаратам этой группы нет противопоказаний [91][367-377].
ЕОК IА (УУР А, УДД 2)
Комментарии. У пациентов с острым ИМбпST титрование дозы иАПФ рекомендуется начать в первые 24 ч от начала лечения после стабилизации гемодинамики. У больных с ИМбпST и/или ФВ ЛЖ ≤40% рекомендуется использовать целевые дозы иАПФ с доказанным положительным влиянием на прогноз, дозу которых следует постепенно увеличивать до рекомендуемой (целевой), а если это невозможно, до максимально переносимой (Приложение А3. Медикаментозное лечение ОКСбпST; Приложение А3. Дозы антитромботических лекарственных средств при нарушенной функции почек).
Противопоказания для начала использования иАПФ: систолическое АД <100 мм рт.ст., выраженное нарушение фильтрационной функции почек, гиперкалиемия, двусторонний стеноз почечных артерий (или стеноз почечной артерии единственной почки), беременность, индивидуальная непереносимость.
- У всех пациентов с ОКСбпST рекомендуются рутинное применение иАПФ для снижения риска сердечно-сосудистых осложнений, если к препаратам этой группы нет противопоказаний [367][368].
ЕОК IIaА (УУР В, УДД 2)
Комментарии. В рандомизированных контролируемых исследованиях у больных без известной ФВ ЛЖ <40% и СН показана польза при использовании рамиприла** и периндоприла**.
- У пациентов с ИМбпST, имеющих СН и/или низкую (≤40%) ФВ ЛЖ в случае непереносимости иАПФ для лечения СН, снижения риска смерти и прогрессирования СН рекомендуется использовать АРА, предпочтительно — валсартан [369][378-380].
ЕОК IB (УУР B, УДД 2)
Комментарии. Начальная доза валсартана составляет 20 мг 2 раза/сут. (40 мг/сут.); при хорошей переносимости дозу препарата постепенно увеличивают вплоть до 160 мг 2 раза/сут.
- У пациентов с ИМбпST и низкой (≤40%) ФВ ЛЖ и/или признаками застоя в легких для снижения риска смерти от сердечно-сосудистых причин и госпитализации по поводу СН рекомендуется назначить валсартан + сакубитрил** [381-392].
ЕОК IIaB (УУР B, УДД 2)
Комментарии. По результатам исследования PARADISE-MI валсартан + сакубитрил** (группа C09DX — АРА в комбинации с другими средствами) не уступал рамиприлу по влиянию на частоту смерти или госпитализации по поводу утяжеления СН при назначении в ранний период (от 12 ч до 7 сут.) ОКСбпST [381-392]. В исследовании PARADIGM-HF валсартан + сакубитрил** продемонстрировал бόльшую эффективность по сравнению с эналаприлом** в отношении снижения риска смерти или госпитализации по поводу утяжеления СН у больных ХСН с низкой ФВ ЛЖ [393].
Антагонисты альдостерона
- У пациентов с ИМбпST при ФВ ЛЖ ≤40% в сочетании с СН или СД, а также у пациентов с НС и сохраняющейся СН II-IV ФК по NYHA при ФВ ЛЖ ≤35%, не имеющих существенного снижения фильтрационной функции почек и гиперкалиемии, к терапевтическим дозам иАПФ и β-АБ рекомендуется добавить антагонист альдостерона, предпочтительно эплеренон, для предотвращения СН и смерти [93][394-396].
ЕОК IIaB (УУР A, УДД 2)
Комментарии. Предпочтительно назначать эплеренон в первые 3 сут. от начала ИМбпST. Противопоказания к назначению антагонистов альдостерона: уровень креатинина в крови у мужчин >220 мкмоль/л, у женщин >175 мкмоль/л, а также концентрация калия >5 ммоль/л. Во время лечения антагонистами альдостерона контролируют уровень креатинина и калия крови. Если уровень калия в крови превышает 5,5 ммоль/л, препараты отменяют. Рекомендуется поддерживать концентрацию калия в крови в диапазоне 4,0-5,0 ммоль/л.
3.2.8. Липидснижающая терапия
- Для снижения суммарного риска повторных ишемических событий у пациентов с ОКСбпST вне зависимости от исходного уровня ХС рекомендуется в период госпитализации начать или продолжить лечение ингибитором 3-гидрокси-3-метилглутарил коэнзима А редуктазы (ингибитором ГМГ-КоА-редуктазы) в высокой дозе при отсутствии противопоказаний [397-401].
ЕОК IA (УУР A, УДД 1)
Комментарии. Назначение (первый прием) ингибитора ГМГ-КоА-редуктазы в высокой дозе рекомендуется выполнить в первые сутки госпитализации пациента с ОКСбпST и при этом как можно ранее. В случае выполнения ЧКВ первый прием ингибитора ГМГ-КоА-редуктазы рекомендуется до его начала с целью снижения вероятности развития сердечно-сосудистых осложнений и контраст-индуцированной нефропатии после ЧКВ [402][403].
И целесообразность назначения, и начальная доза ингибитора ГМГ-КоА-редуктазы при ОКСбпST не определяются уровнем общего ХС и его фракций, поэтому не должно быть задержки в назначении ингибитора ГМГ-КоА-редуктазы из-за ожидания результатов биохимического анализа крови.
Терапия ингибиторами ГМГ-КоА-редуктазы обычно хорошо переносится, частота серьезных побочных эффектов не превышает 2%. Возможно появление миалгии и мышечной слабости, которые появляются в основном в первые недели после начала терапии и обычно быстро проходят после отмены ингибитора ГМГ-КоА-редуктазы. Рабдомиолиз с повышением уровня креатинкиназы более чем в 10 раз наблюдается крайне редко. Возможно бессимптомное повышение уровня трансаминаз в ≥3 раза, что требует снижения дозы или отмены препарата с дальнейшим наблюдением за активностью печеночных трансаминаз. Следует помнить, что в острую фазу ИМбпST повышение уровня трансаминаз обусловлено некрозом кардиомиоцитов и не является причиной для отказа от назначения ингибиторов ГМГ-КоА-редуктазы.
Основные противопоказания для назначения ингибиторов ГМГ-КоА-редуктазы: повышенная чувствительность; активное заболевание печени или повышение активности печеночных трансаминаз в плазме крови неясного генеза более чем в 3 раза по сравнению с верхней границей нормы; женщины детородного возраста, не использующие адекватные методы контрацепции; беременность; период грудного вскармливания.
Рекомендуется применение ингибиторов ГМГ-КоА-редуктазы с наиболее выраженным гиполипидемическим действием, в частности аторвастатина** в суточной дозе 40-80 мг или розувастатина в суточной дозе 20-40 мг.
- Для дополнительного снижения суммарного риска ишемических событий у пациентов с ОКСбпST в период госпитализации рекомендуется рассмотреть начало комбинированного лечения ингибитором ГМГ-КоА-редуктазы в высокой дозе и эзетимибом (вне зависимости от исходного уровня ХС и его фракций и предшествующей терапии ингибитором ГМГ-КоА-редуктазы) [404].
ЕОК IIbB (УУР B, УДД 2)
Комментарии. Эта рекомендация основана на результатах исследования IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). В ранней стадии (в первые 10 дней) ОКС эзетимиб назначали в дополнение к предшествующей терапии статинами или назначали одновременно со статинами у пациентов, ранее не принимавших статины (две трети пациентов), сравнивали с монотерапией статинами. Было показано, что лечение эзетимибом безопасно и обеспечивает долгосрочные преимущества в отношении сердечно-сосудистых исходов [404].
- У пациентов, перенесших ОКСбпST, рекомендуется поддерживать уровень ХС ЛНП <1,4 ммоль/л и при этом добиваться его снижения как минимум на 50% от исходных значений для обеспечения максимального эффекта по снижению риска повторных ишемических событий [134][155][401][404].
ЕОК IA (УУР А, УДД 1)
Комментарии. Необходимо определять уровень ХС ЛНП в крови через 4-6 нед. после начала лечения, после изменения дозы или добавления нового гиполипидемического препарата для оценки эффективности терапии, определения необходимости увеличения дозы и/или добавления гиполипидемических препаратов с другим механизмом действия (эзетимиб и/или алирокумаб** или эволокумаб** (группа C10AX — другие гиполипидемические средства) или инклисиран) [404].
- У пациентов с ОКСбпST, перенесших помимо данного ОКСбпST еще одно сосудистое (в любом сосудистом бассейне) событие в течение предшествующих 2 лет, для дополнительного снижения риска повторных ишемических событий рекомендуется целевой уровень ХС ЛНП <1,0 ммоль/л [51][405].
ЕОК IIbB (УУР С, УДД 5)
- У пациентов, которые на момент развития ОКСбпST уже принимали ингибитор ГМГ-КоА-редуктазы в максимально переносимой дозе и эзетимиб, но при этом при поступлении имеют уровень ХС ЛНП выше целевого, рекомендуется в дополнение к ингибитору ГМГ-КоА-редуктазы в максимально переносимой дозе и эзетимибу раннее назначение (во время госпитализации в связи с данным коронарным событием) блокатора пропротеиновой конвертазы субтилизин-кексинового типа 9 (PCSK9) — алирокумаба**, эволокумаба** или инклисирана [406-409].
ЕОК IIaС для алирокумаба и эволокумаба, РКО IIaС для инклисирана (УУР В, УДД 2)
Комментарии. Клиническая эффективность инклисирана показана в метаанализах относительно небольших исследований, в которые включались в т. ч. больные, перенесшие ИМ. В настоящее время проводится крупномасштабное исследование ORION-4 по изучению влияния инклисирана на сердечно-сосудистые осложнения, результаты ожидаются в 2025г.
- При значительном повышении (>4,0 ммоль/л) уровня ХС ЛНП у пациентов с ОКСбпST рекомендуется раннее назначение (во время госпитализации в связи с данным коронарным событием) ингибитора ГМГ-КоА-редуктазы и эзетимиба [410].
РКО IIаA (УУР А, УДД 1)
- При чрезвычайно высоком (>5,0 ммоль/л) повышении уровня ХС ЛНП у пациентов с ОКСбпST рекомендуется раннее назначение (во время госпитализации в связи с данным коронарным событием) ингибитора ГМГ-КоА-редуктазы в максимально переносимой дозе + эзетимиб + ингибитор PCSK9 (алирокумаб**, эволокумаб** или инклисиран) [411-415].
РКО IIаA (УУР А, УДД 1)
- Если у пациента, перенесшего ОКСбпST, при использовании максимально переносимой дозы ингибитора ГМГ-КоА-редуктазы концентрация ХС ЛНП в крови остаётся выше целевого уровня, рекомендуется добавить к ингибитору ГМГ-КоА-редуктазы эзетимиб для дополнительного снижения уровня ХС ЛНП в крови и риска ишемических событий [404], в т. ч. ингибитор ГМГ-КоА-редуктазы с эзетимибом в одной таблетке или капсуле.
ЕОК IIaB (УУР B, УДД 2)
Комментарии. Данная рекомендация относится к пациентам, у которых во время госпитализации по поводу ОКСбпST эзетимиб не был назначен согласно рекомендации для госпитального этапа.
- Если у пациента, перенесшего ОКСбпST, при использовании максимально переносимой дозы ингибитора ГМГ-КоА-редуктазы в сочетании с эзетимибом концентрация ХС ЛНП в крови остаётся выше целевого уровня, рекомендуется добавить алирокумаб** или эволокумаб** или инклисиран для дополнительного снижения уровня ХС ЛНП в крови и риска ишемических событий [416-418].
ЕОК IB для алирокумаба и эволокумаба, РКО IB для инклисирана (УУР A, УДД 2)
Комментарии. Если на фоне терапии ингибиторами ГМГ-КоА-редуктазы в максимально переносимых дозах уровень ХС ЛНП остается значительно повышенным (>2,5 ммоль/л), можно рассмотреть добавление алирокумаба** или эволокумаба** или инклисирана без предварительного применения эзетимиба.
- У пациентов с ОКСбпST или после ОКСбпST с непереносимостью ингибиторов ГМГ-КоА-редуктазы для достижения целевых значений ХС ЛНП рекомендуется рассмотреть возможность использования эзетимиба и/или препарата из группы блокаторов PCSK9 (алирокумаб** или эволокумаб** или инклисиран) [3].
ЕОК IIbС для алирокумаба и эволокумаба, РКО IIbС для инклисирана (УУР C, УДД 5)
3.3. Иное лечение
- У пациентов с ОКСбпST при концентрации глюкозы в крови >10 ммоль/л рекомендуется использование сахароснижающих лекарственных средств для контроля уровня гликемии [419-422].
ЕОК IIaС (УУР B, УДД 2)
Комментарии. Целевой уровень глюкозы в крови и гликированного гемоглобина при лечении ОКСбпST не определен и должен выбираться с учетом сопутствующих заболеваний. Следует избегать гипогликемии.
Для достижения целевого уровня гликемии у пациентов с ОКСбпST рекомендуется индивидуализированный подход к выбору сахароснижающих лекарственных средств. Наличие у пациента с ОКСбпST СД 2 типа не является обязательным показанием к переводу на инсулинотерапию; многие могут продолжать прием пероральных сахароснижающих препаратов. Если у пациента с СД 2 типа возникла необходимость в использовании инсулинов, после стабилизации состояния рекомендуется переход на пероральные сахароснижающие средства.
Пациенты с ОСКбпST без нарушения сознания и других серьезных осложнений, способные самостоятельно принимать пищу, могут находиться на подкожной инсулинотерапии при условии, что она позволяет поддерживать целевой диапазон гликемии и избегать гипогликемии. Методом выбора для быстрого и управляемого достижения компенсации углеводного обмена является непрерывная в/в инфузия инсулинов и аналогов быстрого действия (Приложение А3. Внутривенная инсулинотерапия при ОКСбпST), при необходимости — в сочетании с в/в инфузией декстрозы**.
- У пациентов с ОКСбпST без СД и при СД вне контроля уровня гликемии не рекомендуется использовать одновременную инфузию инсулинов и декстрозы**, одновременную инфузию инсулинов, декстрозы** и калия хлорида** из-за отсутствия доказательств положительного влияния на смертность и частоту нефатальных осложнений [3][423].
ЕОК IIIC (УУР С, УДД 5)
- У пациентов с ОКСбпST не рекомендуется использовать нестероидные противовоспалительные и противоревматические препараты (за исключением низких доз АСК** в качестве антиагреганта и средних доз ACK** для лечения перикардита) из-за неблагоприятного влияния на прогноз [424][425].
ЕОК IIIB (УУР А, УДД 1)
Комментарии. Рекомендуется отменить нестероидные противовоспалительные и противоревматические средства и/или не начинать их использование при госпитализации с ОКСбпST.
- У пациентов с ОКСбпST и анемией, не имеющих признаков продолжающегося кровотечения и гемодинамической нестабильности, целесообразность гемотрансфузии рекомендуется рассматривать при снижении уровня гематокрита <25% и/или гемоглобина <70 г/л для уменьшения риска осложнений, связанных с гемотрансфузий, и возможного неблагоприятного влияния гемотрансфузий на прогноз [3].
ЕОК IIbC (УУР C, УДД 5)
- Пациентам с ОКСбпST, которые находятся в коме после внегоспитальной или внутригоспитальной остановки кровообращения, рекомендуется контроль температуры тела, включающий постоянный мониторинг центральной температуры и активное предотвращение лихорадки (>37,7 °C), в течение по меньшей мере 72 ч с целью предотвращения осложнений [426-436].
ЕОК IB (УУР В, УДД 3)
- При невозможности контроля температуры тела медикаментозным путем у пациентов с ОКСбпST в коме после остановки кровообращения следует рассмотреть применение аппаратной целенаправленной терморегуляции с помощью специальных устройств поверхностного или эндоваскулярного типа с постоянной обратной связью с целевой температурой 32-37 °C — при наличии технических возможностей [426-436].
ЕОК IIaB (УУР В, УДД 2)
3.4. Осложнения
3.4.1. ОСН
ОСН может возникать как осложнение ОКСбпST или отражать декомпенсацию ранее существовавшей ХСН при ишемическом повреждении миокарда. При возникновении ОСН у пациентов с ОКСбпST увеличивается риск других осложнений: снижения фильтрационной функции почек, дыхательной недостаточности, пневмонии, повышается вероятность летального исхода заболевания.
У пациента с ОСН всегда можно диагностировать дисфункцию миокарда ЛЖ. Степень дисфункции миокарда ЛЖ является независимым предиктором смертности пациента с ОКСбпST. Раннее выявление нарушенной функции миокарда при проведении ЭхоКГ позволяет своевременно начать лечебные мероприятия по профилактике развития СН.
Развитие ОСН у пациентов с ИМбпST ухудшает краткосрочный и долгосрочный прогноз. Классификация Killip была разработана и используется у пациентов с ИМ (в т. ч. ИМбпST) для оценки тяжести ОСН и оценки краткосрочного прогноза.
Основной стратегией терапии у пациентов с ОКСбпST, осложненном ОСН, является экстренная реваскуляризация миокарда с целью ограничения ишемического повреждения.
- У пациентов с ОКСбпST и ОСН рекомендуется непрерывное мониторирование основных жизненно важных функций — как минимум сердечного ритма, АД, SpO2 и диуреза — до стабилизации гемодинамики [3][437][438].
РКО IIaC (УУР С, УДД 5)
- Пациентам с ОКСбпST при появлении признаков ОСН рекомендуется срочно выполнить ЭхоКГ с целью оценки сократительной функции миокарда ЛЖ, состояния клапанов и поиска механических осложнений [3].
РКО IIaC (УУР С, УДД 5)
Комментарии. В случае, если ЭхоКГ уже была выполнена, рекомендуется повторная ЭхоКГ для контроля за общей и локальной сократительной функцией ЛЖ и выявления/исключения механических осложнений ИМбпST. Также рекомендуется контроль ЭхоКГ в динамике для оценки эффективности и безопасности проводимой терапии.
- Для оценки наличия и выраженности ОСН рекомендуется во всех случаях ИМбпST использовать классификацию Killip [3][439][440].
ЕОК IIaC (УУР C, УДД 5)
Комментарии. Классификация Killip приведена в Приложении А3. Классификация ОСН при ИМ по Killip.
3.4.1.1. Отек легких
На фоне выраженного снижения систолической функции миокарда ЛЖ и/или механических осложнений происходит повышение давления крови в капиллярах малого круга и поступление жидкой компоненты крови из внутрисосудистого русла в ткань легких. Различают интерстициальный и альвеолярный отек легких. Диагностика отека легких в большинстве случаев не вызывает затруднений за счет типичной клинической картины. При осмотре наиболее характерными признаками являются: тахикардия, тахипноэ, вынужденное возвышенное положение (ортопноэ). При аускультации легких выслушиваются "застойные" мелкопузырчатые хрипы. Регистрируется снижение оксигенации крови. Диагноз можно подтвердить с помощью обзорной рентгенографии грудной клетки и УЗИ легких. У пациентов с ОКСбпST и ОСН (особенно при отеке легких) отмечается снижение сатурации крови.
- Рекомендуется ингаляторное введение кислорода (оксигенотерапия) пациенту с ОКСбпST и ОСН путем ингаляции увлажненного кислорода через маску при SpO2 ниже 90% или РaO2 <60 мм рт.ст. с целью коррекции гипоксемии [3][215][217].
ЕОК IC (УУР С, УДД 5)
Комментарии. При длительно проводимом ингаляторном введении кислорода (оксигенотерапии) рекомендуется периодическая оценка газового состава артериальной и венозной крови (исследование кислотно-основного состояния и газов крови).
Рутинное проведение оксигенотерапии пациентам без гипоксемии (SpO2 ≥90%) не рекомендуется.
- Для улучшения эффективности лечения СН у пациентов с ОКСбпST при тяжелой дыхательной недостаточности (SpO2 <90% и тахипноэ >25 в мин) рекомендуется рассмотреть возможность проведения неинвазивной масочной искусственной вентиляции легких (ИВЛ) с постоянным положительным давлением в конце выдоха — постоянной или бифазной вентиляции (неинвазивная ИВЛ, неинвазивная вентиляция с двухуровневым положительным давлением) [441-443].
ЕОК IIaB (УУР А, УДД 1)
Комментарии. Применение неинвазивной вентиляции легких целесообразно с применением маски (continuous positive airway pressure — CPAP или biphasic positive airway pressure — BiPAP). Создание положительного давления в дыхательных путях защищает альвеолы от коллабирования на выдохе и поддерживает объем дыхательной поверхности при отеке легких. В результате уменьшается работа дыхательных мышц, улучшается газообмен в легких и повышается внутригрудное давление со снижением пред- и постнагрузки. Имеются данные, что проведение неинвазивной масочной ИВЛ следует рассмотреть как можно скорее, т. к. она снижает вероятность необходимости интубации трахеи и проведение инвазивной ИВЛ.
Основным режимом неинвазивной вентиляции легких в данной клинической ситуации является CPAP, однако у пациентов с сопутствующей бронхолегочной патологией и гиперкапнией целесообразно использовать BiPAP.
При проведении неинвазивной ИВЛ рекомендуется периодическая оценка газового состава артериальной и венозной крови.
- При наличии у пациента с ОКСбпST дыхательной недостаточности, сопровождающейся выраженной гипоксемией, гиперкапнией и ацидозом, при неэффективности оксигенотерапии и неинвазивной ИВЛ рекомендуется интубация трахеи и проведение инвазивной ИВЛ [441].
ЕОК IC (УУР С, УДД 5)
- У пациентов с ОКСбпST и ОСН с признаками задержки жидкости рекомендуется в/в введение "петлевых" диуретиков [444][445].
ЕОК IC (УУР С, УДД 5)
Комментарии. Используют в/в болюсное введение фуросемида** (группа C03CA —сульфонамиды). Рекомендуемая первоначальная доза — 40 мг. При развернутой картине альвеолярного отека легких, признаках задержки жидкости в организме, тяжелом нарушении фильтрационной функции почек начальная доза фуросемида** может быть увеличена до 60-80 мг. При недостаточной эффективности начальной дозы при повторном введении фуросемида** доза может быть увеличена в 2 раза и более.
- У пациента с ОКСбпST и ОСН с признаками задержки/застоя жидкости при отсутствии адекватного ответа на петлевые диуретики в нарастающих дозах с целью стабилизации состояния рекомендуется рассмотреть целесообразность назначения комбинации "петлевых" диуретиков с тиазидными диуретиками (тиазидами) [445].
ЕОК IIaB (УУР С, УДД 5)
- У пациентов с ОКСбпST и ОСН с признаками застоя жидкости в малом круге кровообращения и систолическим АД ≥110 мм рт.ст., и особенно при высоких цифрах АД, для уменьшения выраженности симптомов ОСН рекомендуется рассмотреть возможность в/в инфузии вазодилататоров (группа C01D — вазодилататоры для лечения заболеваний сердца) [446-450].
ЕОК IIbB (УУР С, УДД 5)
Комментарии. У пациентов с отеком легких при повышенном АД нитроглицерин** является препаратом первого ряда. При исходных значениях систолического АД <90 мм рт.ст. или признаках гипоперфузии, а также поражении ПЖ, введение нитроглицерина противопоказано.
- Рутинное назначение опиоидных препаратов при развитии ОСН у пациента с ОКСбпST не рекомендуется за исключением пациентов с тяжелым болевым синдромом или возбуждением [451-454].
ЕОК IIIC (УУР С, УДД 4)
Комментарии. Ретроспективный анализ показал, что использование опиатов ассоциировано с увеличением потребности в ИВЛ, увеличением сроков госпитализации, увеличением неблагоприятных исходов в стационаре.
3.4.1.2. Кардиогенный шок
Кардиогенный шок — самая тяжелая форма ОСН у пациентов с ОКСбпST. Кардиогенный шок является жизнеугрожающим состоянием, вызванным резким снижением сердечного выброса и проявляющимся признаками выраженной гипоперфузии органов и тканей и тканевой гипоксией, что проявляется клинически: снижением температуры кожных покровов и их мраморностью, снижением темпа диуреза (<40 мл/ч), изменениями психического статуса и сознания.
Выделяют 3 основных патогенетических варианта кардиогенного шока:
- Истинный кардиогенный шок, связанный со снижением сократительной способности ЛЖ;
- Шок вследствие относительной и абсолютной гиповолемии. Близкий к этому вариант — рефлекторный шок, связанный с реакцией на болевой приступ;
- Аритмический вариант — нарушения гемодинамики на фоне тяжелых тахи- и брадиаритмий (см. Раздел "Нарушения ритма").
Частота различных причин кардиогенного шока, по данным регистра SHOCK: у 79% — снижение сократительной функции ЛЖ, 7% — острая митральная недостаточность, 4% — разрыв МЖП, 2% — изолированная правожелудочковая недостаточность, 1,4% — тампонада, 7% — другие причины (осложнения катетеризации, передозировка или неоправданное назначение β-АБ, блокаторов медленных кальциевых каналов и др.) [455].
Основным клиническим признаком кардиогенного шока является стойкая гипотензия (систолическое АД ≤90 мм рт.ст.), рефрактерная к инфузионной терапии и сопровождающаяся признаками острой полиорганной недостаточности в результате гипоперфузии. Объективизация параметров гемодинамики с помощью инвазивных методик, в т. ч. установки катетера Свана-Ганса, на сегодняшний день не считается рутинно обязательной, однако в спорных и сложных случаях позволяет получить ряд ценных данных.
Гемодинамические критерии истинного шока:
- Гипотензия — систолическое АД <80-90 мм рт.ст. при среднем АД <70 мм рт.ст. Формула расчета среднего АД = 1/3 систолического АД + 2/3 диастолического АД.
- Резкое снижение сердечного индекса (СИ) по данным инвазивного мониторинга гемодинамики:
— <1,8 л/мин/м² без вазоактивной терапии;
— <2,0-2,2 л/мин/м² на фоне вазоактивной терапии.
- Повышение давления наполнения:
— Конечное диастолическое давление (КДД) ЛЖ >18 мм рт.ст.;
— КДД ПЖ >10 мм рт.ст.
С точки зрения инвазивной гемодинамики кардиогенный шок характеризуется сниженным СИ, повышением общего периферического сопротивления сосудов и давления заклинивания легочных капилляров (ДЗЛК). Однако эти показатели могут быть и иными. Основываясь на данных инвазивного мониторинга гемодинамики, можно выделить гемодинамические варианты кардиогенного шока (Приложение А3. Гемодинамические варианты кардиогенного шока).
- Пациентам с ОКСбпST и кардиогенным шоком рекомендована установка центрального венозного катетера (катетеризация подключичной и других центральных вен) с целью введения лекарственных препаратов, прежде всего, вазоактивных препаратов, измерения смешанной венозной сатурации и ЦВД (Приложение А3. Параметры для мониторирования у пациента с кардиогенным шоком) [444][456][457].
РКО IIaC (УУР С, УДД 5)
- Пациентам с ОКСбпST и кардиогенным шоком рекомендуется клинический осмотр не реже 1 раза в час и мониторирование жизненно важных функций с целью определения клинической тяжести и реакции на лечение, с кратностью, рекомендованной в Приложении А3. Параметры для мониторирования у пациента с кардиогенным шоком [444][456][457].
РКО IIaC (УУР С, УДД 5)
В зависимости от клинической тяжести и реакции на лечение выделяют следующие стадии кардиогенного шока: прешок, развернутая картина кардиогенного шока и резистентный кардиогенный шок (Приложение А3. Стадии кардиогенного шока в зависимости от клинической тяжести и реакции на лечение), которые также могут быть разбиты на пять стадий согласно классификации Американского общества сердечно-сосудистой ангиографии и интервенции (SCAI) (Приложение А3. Стадии кардиогенного шока согласно классификации Американского общества сердечно-сосудистой ангиографии и интервенции (SCAI)).
Реперфузионная терапия и реваскуляризация миокарда является основой лечения кардиогенного шока, ассоциированного с ОКСбпST.
Лечение истинного кардиогенного шока
- При появлении признаков наличия острой левожелудочковой недостаточности/кардиогенного шока у пациентов с ОКСбпST рекомендуется срочная реваскуляризация миокарда, если она ранее не проводилась или была неполной [3][149][198].
ЕОК IА (УУР В, УДД 2)
Комментарии. См. также Раздел "Инвазивная стратегия лечения у пациентов с кардиогенным шоком и внезапной остановкой кровообращения".
- У пациентов с ОКСбпST и кардиогенным шоком рекомендуется рассмотреть возможность проведения инвазивного мониторинга показателей центральной гемодинамики для контроля за эффективностью и безопасностью лечения, а также катетеризации легочной артерии — для уточнения причины кардиогенного шока, при наличии физических и технических возможностей [3].
РКО IIaC (УУР С, УДД 5)
- У пациентов с ОКСбпST и ОСН с систолическим АД <90 мм рт.ст. и признаками гипоперфузии для устранения гемодинамической нестабильности рекомендуется рассмотреть возможность назначения инотропных препаратов [444][458].
ЕОК IIbC (УУР C, УДД 5)
Комментарии. Инфузия допамина** (группа C01CA — адренергические и дофаминергические средства) начинается со скоростью 5 мкг/кг/мин, далее в зависимости от гемодинамического ответа может быть увеличена до 15-20 мкг/кг/мин. Добутамин** (группа C01CA — адренергические и дофаминергические средства) вводят с начальной скоростью 2-10 мкг/кг/мин; при необходимости скорость может быть увеличена до 20 мкг/кг/мин. Норэпинефрин** (группа C01CA — адренергические и дофаминергические средства) начинают вводить со скоростью 2 мкг/кг/мин, при необходимости скорость увеличивают. При недостаточном эффекте препараты можно комбинировать, прежде всего добутамин** и норэпинефрин**.
У пациентов c ОКСбпST и ОСН, длительно получавших до развития ОКСбпST β-АБ, и у пациентов с недостаточным выделением мочи в ответ на повторное в/в введение диуретиков в качестве альтернативы адренергическим агентам может рассматриваться левосимендан** (группа C01CX — другие кардиотонические средства). Инфузия левосимендана** начинается с нагрузочной дозы (6 мкг/кг), введенной в/в болюсом за 10 мин, с последующей в/в инфузией в дозе 0,1 мг/кг/мин и титрацией до 0,2 мг/кг/мин (до стабилизации систолического АД в течение не менее чем 3-х часов). Рекомендуемая продолжительность инфузии составляет 24 ч. У пациентов с систолическим АД <100 мм рт.ст. и/или с диастолическим АД <60 мм рт.ст. начальный болюс левосимендана** обычно не вводится для предотвращения гипотонии.
- У пациентов с ОКСбпST и ОСН с систолическим АД <90 мм рт.ст. без признаков гипоперфузии рутинное применение инотропных препаратов не рекомендуется, т. к. потенциальная польза меньше потенциального риска [456][458][459].
ЕОК IIIC (УУР С, УДД 4)
- У пациентов с ОКСбпST и кардиогенным шоком для устранения гемодинамической нестабильности и поддержания кровоснабжения жизненно важных органов при артериальной гипотонии рекомендуется рассмотреть возможность назначения вазопрессоров, предпочтительного использования норэпинефрина** (в/в инфузия) по сравнению с допамином** в связи с меньшей частотой побочных действий эффектов норэпинефрина** [460-462].
ЕОК IIbB (УУР А, УДД 2)
- У пациентов с ОКСбпST и кардиогенным шоком при наличии технической возможности рекомендуется рассмотреть возможность использования внешних и внутренних устройств для поддержки кровообращения: внутриаортальная баллонная контрпульсация (ВАБК), устройство вспомогательного кровообращения с постоянным кровотоком левое предсердие-аорта, устройство вспомогательного кровообращения с постоянным кровотоком ЛЖ-аорта, устройство вспомогательного кровообращения с пульсирующим кровотоком ЛЖ-аорта, экстракорпоральная мембранная оксигенация (ЭКМО) [3][444][463].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. Этот подход используется как временная мера ("мост") для стабилизации состояния пациентов, готовящихся к реваскуляризации, хирургическому устранению причин шока, трансплантации сердца или принятию другого промежуточного решения.
- У пациентов с ОКСбпST и кардиогенным шоком рекомендуется рассмотреть возможность использования ВАБК, если такое лечение является мостом к реваскуляризации, трансплантации сердца, установке системы длительной механической поддержки кровообращения или принятию другого промежуточного решения [457].
ЕОК IIbC (УУР С, УДД 5)
- Рутинное применение системы ВАБК у пациентов с ОКСбпST и кардиогенным шоком не рекомендовано, если это лечение не является мостом к другому типу лечения ОСН и/или ее причины [3][464-467].
ЕОК IIIC (УУР С, УДД 5)
Лечение гиповолемического кардиогенного шока
- Всем пациентам с ОКСбпST и подозрением на гиповолемический шок рекомендуется ЭхоКГ для выявления возможных причин гиповолемии (инфаркт ПЖ, тампонада сердца и т. д.) и оценки функционального состояния сердца [3][468].
ЕОК IIbC (УУР С, УДД 5)
- У пациентов с ОКСбпST и признаками гиповолемического шока для обеспечения эффективности и безопасности лечение рекомендуется проводить под контролем показателей центральной гемодинамики, при наличии технических возможностей — с измерением давления ДЗЛК, используя катетер Сван-Ганcа (катетер баллонный для легочной артерии, стандартный) [3][468].
ЕОК IIbC (УУР С, УДД 5)
Комментарии. При отсутствии возможности измерять ДЗЛК, возможна оценка ЦВД, но следует учитывать, что у пациентов с ОКСбпST показатели ЦВД могут не отражать КДД в ЛЖ.
- Пациентам с ОКСбпST и гиповолемическим шоком возмещение жидкости с целью нормализации АД рекомендуется начать с в/в введения 200-250 мл раствора натрия хлорида** 0,9%, который вводят за 5-10 мин. При сохранении артериальной гипотонии рекомендуется вводить раствора натрия хлорида** 0,9% повторно [3][468].
ЕОК IIbC (УУР С, УДД 5)
Комментарии. Объем переливаемой пациенту жидкости может доходить до нескольких литров. При стабилизации АД, появлении диуреза инфузию прекращают. Если введение жидкости не дает эффекта, параллельно проводят лечение адрено- и допаминостимуляторами — допамином** или добутамином**.
3.4.2. Нарушения сердечного ритма и проводимости
3.4.2.1. ЖА
- У пациентов с ОКСбпST, осложненном гемодинамически значимой ЖТ или ФЖ, для восстановления кровообращения рекомендуется проведение немедленной наружной электрической кардиоверсии (дефибрилляции), соответственно [3].
РКО IC (УУР С, УДД 5)
- У пациентов с ОКСбпST и рецидивирующими ЖТ/ФЖ с целью устранения ишемии как возможной причины возникновения аритмий (в случае, если реваскуляризация ранее не проводилась или была неполной) рекомендуется рассмотреть выполнение экстренной и/или полной реваскуляризации (см. Раздел Инвазивное лечение заболевания) [3][73][469][470].
ЕОК IC (УУР С, УДД 5)
Комментарии. Особенно важно выполнение полной реваскуляризации у пациентов с ОКСбпST с ЖТ/ФЖ при сниженной глобальной систолической функции ЛЖ. В случае отсутствия эффективной сердечной деятельности КГ и ЧКВ могут проводиться на фоне механических компрессий грудной клетки при помощи специальных устройств — автоматических приспособлений для непрямого массажа сердца.
- У пациентов c ОКСбпST c полиморфной ЖТ или ФЖ при отсутствии противопоказаний рекомендуется в/в введение β-АБ и/или амиодарона** [340][471-474].
ЕОК IB (УУР В, УДД 3)
Комментарии. Не рекомендуется раннее в/в введение β-АБ при гипотензии, кардиогенном шоке, брадиаритмии, подозрении на вовлеченность в очаг ишемии ПЖ. Перед началом использования β-АБ следует оценить сократительную способность ЛЖ по ЭхоКГ; в случае сниженной сократительной способности ЛЖ целесообразно использование малых доз или отложить инициацию терапии. При выборе конкретного β-АБ, пути его введения и при титрации дозы рекомендуется оценивать в динамике состояние пациента, переносимость терапии, достигнутый эффект и наличие ограничивающих факторов: ЧСС <50 уд./мин, АД <90 мм рт.ст., величину ФВ ЛЖ по данным ЭхоКГ и др.
При увеличении продолжительности интервала QT >500 мс введение амиодарона** (группа C01BD — антиаритмические препараты, класс III) должно быть прекращено.
- У пациентов с ОКСбпST c рецидивирующей ЖТ и отсутствием эффекта от терапии β-АБ и амиодароном** или в случаях, когда эти препараты не могут быть назначены, рекомендуется рассмотреть в/в введение лидокаина** [3][340].
ЕОК IIbC (УУР C, УДД 5)
Комментарии. Лидокаин** — группа C01BB — антиаритмические препараты, класс IB.
- У пациентов с ОКСбпST и рецидивирующей жизнеугрожающей ЖТ при отсутствии эффекта от терапии β-АБ или амиодароном** рекомендовано рассмотреть возможность седации или полной анестезии с целью снижения симпатического тонуса [73].
ЕОК IIbC (УУР C, УДД 5)
Комментарии. Такой метод может рассматриваться только у отдельных пациентов, т. к. могут повышаться риски воспалительных и тромботических осложнений [73].
- У пациентов с ОКСбпST при развитии ЖТ и/или ФЖ рекомендуется мониторинг, выявление и коррекция электролитных нарушений (в первую очередь гипокалиемии и гипомагниемии) [3].
РКО IIaC (УУР С, УДД 5)
Комментарии. С целью выявления электролитных нарушений проводится исследование уровня калия в крови, исследование уровня общего магния в сыворотке крови, исследование кислотно-основного состояния и газов крови.
Введение препаратов калия необходимо, если его уровень в крови <4 ммоль/л. Примерно у 40% больных одновременно с гипокалиемией обнаруживают снижение концентрации магния. У этих больных особенно высок риск ФЖ. Следовательно, у пациентов с ОКСбпST целесообразно определять уровень магния. Если концентрация магния составит <0,83 ммоль/л (2 мг/дл), следует провести коррекцию.
Для коррекции гипокалиемии применяют в/в калия хлорид**, для коррекции уровня магния применяют в/в магния сульфат**; дозу, схему и длительность определяют индивидуально, в зависимости от концентрации электролитов в крови, показателей кислотно-щелочного баланса и клинической ситуации.
- У пациентов с ОКСбпST c рецидивирующей ЖТ/ФЖ и нестабильной гемодинамикой, несмотря на медикаментозную коррекцию, рекомендуется рассмотреть возможность проведения механической поддержки кровообращения — при наличии физических и технических возможностей [340].
ЕОК IIbC (УУР С, УДД 5)
- У пациентов с ОКСбпST и рецидивирующей несмотря на реваскуляризацию и оптимальную медикаментозную терапию ЖТ и/или ФЖ рекомендуется выполнение в подострую стадию радиочастотной аблации аритмогенных зон с последующей имплантацией кардиовертера-дефибриллятора (КВД) [3].
ЕОК IIaC (УУР С, УДД 5)
- У пациентов с ОКСбпST с устойчивой ЖТ или ФЖ, возникшей позднее 48 ч от начала ОКСбпST, при отсутствии данных за сохраняющуюся ишемию миокарда рекомендуется имплантация КВД — при наличии технической возможности [340].
ЕОК IА (УУР С, УДД 5)
- Пациентам, перенесшим ИМбпST (не менее 6 нед. назад) при наличии у них несмотря на оптимальную медикаментозную терапию (в течение 3 мес. или более) симптомной ХСН (II-III ФК по NYHA) и ФВ ЛЖ ≤35%, при ожидаемой продолжительности жизни не менее 1 года и сохранном функциональном статусе пациента рекомендуется имплантация КВД (при наличии показаний — в сочетании с ресинхронизирующей терапией) [475][476].
ЕОК IА (УУР А, УДД 1)
Комментарии. Критериев определения ожидаемой продолжительности жизни не разработано. Решение рекомендуется принимать индивидуально, с учетом особенностей каждого клинического случая. О малой ожидаемой продолжительности жизни свидетельствуют, в частности, поздние стадии злокачественного новообразования с метастазами и отсутствием эффекта от проводимого лечения, паллиативное лечение тяжелой ХСН.
- У пациентов с полиморфной ЖТ или ФЖ, возникшей позднее 48 ч от начала ИМбпST, при условии неполной реваскуляризации миокарда, исходно низкой ФВ ЛЖ, рекомендуется рассмотреть возможность имплантации КВД или использования носимого КВД в период до 40 дней от развития ИМбпST [3].
ЕОК IIbC (УУР C, УДД 5)
- У пациентов с ОКСбпST не рекомендуется лечение асимптомных и гемодинамически незначимых ЖА с помощью антиаритмических препаратов [3][340].
ЕОК IIIС (УУР С, УДД 5)
- У пациентов с ОКСбпST не рекомендуется профилактическое назначение антиаритмических препаратов за исключением β-АБ [340].
ЕОК IIIB (УУР С, УДД 5)
3.4.2.2. ФП
ФП — наиболее частый тип наджелудочковой аритмии, встречающийся у пациентов с ОКСбпST. ФП, как правило, хорошо переносится, но у ряда пациентов на фоне тахисистолии может развиться гемодинамическая нестабильность. У гемодинамически стабильных пациентов, как правило, специфического лечения не требуется, за исключением антикоагулянтной профилактики тромбоэмболических осложнений.
- У пациентов с ОКСбпST и недавно возникшей ФП при гемодинамической нестабильности рекомендуется немедленное проведение наружной электрической кардиоверсии [3].
ЕОК IС (УУР С, УДД 5)
- У гемодинамически нестабильных пациентов с ОКСбпST и недавно возникшей ФП для поддержки наружной электрической кардиоверсии и снижения риска рецидива ФП рекомендуется использование амиодарона** в/в [3][477][478].
ЕОК IC (УУР C, УДД 5)
Комментарии. При увеличении продолжительности интервала QT >500 мс введение амиодарона** должно быть прекращено. Введение амиодарона** у нестабильных пациентов должно проводиться под мониторным контролем параметров гемодинамики.
- У пациентов с ОКСбпST и тахисистолической формой ФП при наличии ОСН, но отсутствии тяжелой гипотонии рекомендуется в/в введение амиодарона** с целью контроля ЧСС [3][310][479].
ЕОК IС (УУР С, УДД 5)
Комментарии. Необходим контроль продолжительности интервала QT; если продолжительность интервала QT превышает 500 мс, введение амиодарона** сразу прекращают.
- У пациентов с ОКСбпST и ФП при отсутствии ОСН и гемодинамической нестабильности (тяжелой гипотонии) рекомендуется использовать β-АБ в/в, если это необходимо для контроля ЧСС [3][480].
ЕОК IC (УУР С, УДД 5)
- У пациентов с ОКСбпST и впервые возникшей в острой фазе заболевания ФП рекомендуется рассмотреть вопрос о назначении оральных антикоагулянтов на неопределенно долгий срок, с переоценкой всей антитромботической терапии — в зависимости от риска развития тромбоэмболических осложнений по шкале CHA2DS2-VASc и риска кровотечений по шкале HAS-BLED [3][481-483].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. Необходимость коррекции сроков двойной антитромбоцитарной терапии и монотерапии антиагрегантом в составе антитромботической терапии у пациентов с ОКСбпST и ФП определяется соотношением риска тромбоэмболических, ишемических и геморрагических осложнений и представлена в Разделе "Антитромботическая терапия при других стратегиях лечения у пациентов с наличием показаний к длительному пероральному приему антикоагулянтов".
Предпочтительно использовать прямые оральные антикоагулянты (АТХ-группы Прямые ингибиторы тромбина, В01АЕ и Прямые ингибиторы фактора Ха, В01АF), если к ним нет противопоказаний.
3.4.2.3. Синусовая брадикардия, АВ-блокада
- У пациентов с ОКСбпST при развитии гемодинамически значимой синусовой брадикардии или гемодинамически значимой АВ-блокады (с неадекватным замещающим ритмом) для стабилизации гемодинамики рекомендуется в/в введение препаратов, оказывающих положительное хронотропное действие (эпинефрина**, атропина**) [3][484][485].
ЕОК IC (УУР С, УДД 5)
Комментарии. При ЧСС 40 уд./мин и/или паузах >300 мс вводят атропин** 0,6-1,0 мг. Эпинефрин** — группа C01CA — адренергические и дофаминергические средства. При сохранении выраженной брадикардии устанавливают ЭКС.
- У пациентов с ОКСбпST c гемодинамически значимой АВ-блокадой (с неадекватным замещающим ритмом) при неэффективности атропина** для стабилизации состояния пациента рекомендуется временная эндокардиальная кардиостимуляция — при наличии физических и технических возможностей [3].
ЕОК IC (УУР С, УДД 5)
- У пациентов с ОКСбпST при развитии гемодинамически значимой синусовой брадикардии или гемодинамически значимой АВ-блокады (с неадекватным замещающим ритмом), если ранее реваскуляризация не была проведена или она была неполной, рекомендуется неотложная КГ с намерением выполнения ЧКВ, в т. ч. объеме полной реваскуляризации [3].
ЕОК IC (УУР С, УДД 5)
- У пациентов с ОКСбпST при наличии общих для популяции показаний к имплантации постоянного ЭКС рекомендуется выполнить процедуру имплантации постоянного ЭКС, у пациентов с ИМбпST в срок не ранее 5 дня от его развития [3][486].
ЕОК IC (УУР С, УДД 5)
Комментарии. Решение вопроса об имплантации постоянного ЭКС следует проводить на 10-14 день от развития ИМбпST. Более ранняя (начиная с 5-го дня от начала заболевания) имплантация ЭКС целесообразна у пациентов с гемодинамически значимой брадикардией и поздней и/или неполной реваскуляризацией при ИМбпST передней локализации, при наличии бифасцикулярного блока; при наличии АВ-блокады до развития ИМбпST; с прогрессированием степени АВ-блокады в первые сутки заболевания.
- У отдельных пациентов с ИМбпST передней локализацией, с АВ-блокадой высокой степени и выраженной СН при наличии технической возможности рекомендуется рассмотреть возможность раннего начала ресинхронизирующей терапии [3][457].
ЕОК IIbC (УУР С, УДД 5)
Комментарии. Решение вопроса о начале ресинхронизирующей терапии рекомендуется принимать с учетом наличия у пациента с АВ-блокадой внутрижелудочковой блокады, её выраженности, клинической выраженности СН и степени систолической дисфункции ЛЖ.
- Не рекомендуется проведение кардиостимуляции пациентам с ИМбпST и АВ-блокадой высокой степени в случаях, когда АВ-блокада купировалась после реваскуляризации или спонтанно [3][487][488].
ЕОК IIIB (УУР С, УДД 5)
3.4.3. Механические осложнения
Механические осложнения ИМ: разрыв свободной стенки ЛЖ, разрыв МЖП, острая митральная регургитация вследствие ишемического повреждения структур клапанного аппарата — являются жизнеугрожающими состояниями, которые в реперфузионную эру встречаются с частотой <0,1% случаев у пациентов с ИМбпST.
Выбор оптимальной стратегии ведения пациентов с механическими осложнениями ИМ осуществляется мультидисциплинарной врачебной командой, с участием кардиолога, анестезиолога, рентгенэндоваскулярного хирурга и сердечно-сосудистого хирурга.
- У пациентов с ИМбпST при наличии механических осложнений с гемодинамической нестабильностью рекомендуется использование системы ВАБК*** и/или других механических средств для вспомогательного кровообращения в качестве временной меры для стабилизации состояния до хирургического вмешательства [3][489-492].
ЕОК IIaC (УУР С, УДД 5)
Разрыв свободной стенки ЛЖ
- При доказанном разрыве свободной стенки ЛЖ у пациента с ИМбпST с развитием тампонады сердца или нарастанием признаков её развития рекомендуются немедленная пункция и катетеризация перикарда (син.: перикардиоцентез) с последующим экстренным хирургическим вмешательством [3][489-492].
РКО IIaC (УУР С, УДД 5)
Разрыв МЖП
- У пациентов с ИМбпST при выявлении разрыва МЖП для предотвращения летального исхода рекомендуется хирургическое лечение (закрытие дефекта перегородки сердца) [3][489-492].
РКО IIaC (УУР С, УДД 5)
Комментарии. Способ и время вмешательства зависят от характера дефекта и состояния пациента. В целом хирургическая реконструкция МЖП предпочтительна после стабилизации состояния, однако при выраженном нарушении гемодинамики операция проводится по экстренным показаниям.
Острая митральная регургитация
Острая митральная регургитация при ОКСбпST может быть следствием ишемии и дисфункции папиллярный мышц. При ИМбпST она обычно развивается на 2-7 день вследствие разрыва папиллярной мышцы или хорды. Разрыв может быть полным или включать только отдельные головки мышцы. Наиболее часто происходит разрыв заднемедиальной мышцы вследствие особенностей ее кровоснабжения. В некоторых случаях выраженная митральная регургитация развивается у пациентов с ОКСбпST без разрыва или инфаркта сосочковой мышцы как следствие обширного поражения ЛЖ с его последующей дилатацией и расширением митрального кольца. Клинически острая митральная недостаточность проявляется ОСН, отеком легких, иногда кардиогенным шоком. Диагноз подтверждается на основании данных ЭхоКГ.
- У пациентов с ИМбпST при развитии острой митральной недостаточности для устранения ОСН и предотвращения смерти рекомендуется хирургическое лечение — как правило, протезирование митрального клапана (протезирование митрального клапана в условиях искусственного кровообращения; пластика митрального клапана в условиях искусственного кровообращения; реконструкция подклапанных структур митрального клапана). Время вмешательства зависит от степени митральной регургитации и состояния пациента. В большинстве случаев операция проводится по экстренным показаниям [3][490][491][493][494].
ЕОК IIaC (УУР В, УДД 3)
Комментарии. Время вмешательства зависит от степени митральной регургитации и состояния пациента. В большинстве случаев операция проводится по экстренным показаниям.
- У пациентов с ИМбпST при развитии острой митральной недостаточности в качестве временной меры для стабилизации состояния рекомендуется рассмотреть возможность использования системы ВАБК и/или других механических средств для вспомогательного кровообращения [3][490][491].
ЕОК IIbC (УУР С, УДД 5)
3.4.4. Перикардит
Перикардит может осложнять ранний период ИМбпST (от нескольких часов до 4-х сут.) и обычно быстро проходит самостоятельно (ранний постинфарктный перикардит, син.: эпистенокардитический перикардит) или развиваться в более поздний период (через 1-2 нед., иногда симптомы могут длиться несколько недель) — поздний постинфарктный перикардит, син.: синдром Дресслера. Как правило, вероятность развития перикардита находится в прямой зависимости от объема поражения миокарда и не имеет самостоятельного прогностического значения. Классический синдром Дресслера в виде развернутого полисерозита в последнее время встречается крайне редко.
Клинически перикардит проявляется распространенной, без четкой локализации болью в прекардиальной области, аускультативно — шумом трения перикарда (выслушивается лишь у каждого третьего пациента, нередко в течение короткого времени; при накоплении экссудата в полости перикарда исчезает). На ЭКГ появляются конкордантные подъемы сегмента ST, иногда в сочетании с депрессией сегмента PQ. Перикардит может сопровождаться снижением амплитуды зубцов QRS во всех отведениях ЭКГ. Выявление жидкости в перикарде, так же как и контроль за изменением ее количества, осуществляется с помощью ЭхоКГ.
- У пациентов с ИМбпST и ранним постинфарктным перикардитом с выраженными клиническими проявлениями в качестве противовоспалительной терапии рекомендуется применение #АСК** в дозе 500 мг каждые 8-12 ч [3][495][496].
РКО IIaC (УУР С, УДД 5)
Комментарии. Длительность лечения обычно не превышает 5-7 дней. Использование других, альтернативных АСК**, нестероидных противовоспалительных средств, при постинфарктном перикардите не оправдано из-за потенциального отрицательного влияния на риск сердечных событий.
- У пациентов с ИМбпST и поздним постинфарктным перикардитом с выраженными клиническими проявлениями в качестве противовоспалительной терапии рекомендуется применение #АСК** в дозе 500-1000 мг каждые 6-8 ч (максимальная суточная доза — 3000 мг) с последующим постепенным снижением дозы на 250-500 мг каждые 2 нед. [3][495][496].
РКО IIaC (УУР С, УДД 5)
- В качестве дополнительного средства лечения постинфарктного перикардита возможно использование #безвременника осеннего семян экстракта (колхицина) в течение 3 мес. в дозе 0,5 мг 2 раза/сут. (у пациентов с массой тела ≥70 кг) или 0,5 мг 1 раз/сут. (у пациентов с массой тела <70 кг) [3][495][496].
РКО IIaC (УУР С, УДД 5)
3.4.5. Острая аневризма ЛЖ и тромбоз ЛЖ
Острая аневризма ЛЖ обычно развивается при обширном ИМбпST передней стенки ЛЖ, особенно в отсутствие адекватной реперфузионной терапии. При острой аневризме ЛЖ увеличивается вероятность разрыва сердца, а также таких осложнений, как СН, нарушения ритма сердца, перикардит, тромбоз полости ЛЖ, периферические тромбоэмболии.
Практически всегда при аневризме сердца не менее чем в половине случаев обширных передних ИМбпST обнаруживают пристеночный тромбоз в полости ЛЖ. В популяции пациентов с распространенным передним ИМпST тромбоз ЛЖ встречается более чем в 9% случаев. Методом диагностики острой аневризмы ЛЖ и тромбоза в полости ЛЖ является ЭхоКГ.
- Пациентам с ИМбпST при высокой клинической вероятности тромба в полости ЛЖ, но сомнительных результатах ЭхоКГ для подтверждения/исключения тромба в полости ЛЖ рекомендуется МРТ сердца — при наличии физических и технических возможностей [3][100].
ЕОК IIaC (УУР С, УДД 5)
- У пациентов с передним ИМбпST в случаях нечеткой визуализации тромба в полости ЛЖ при рутинной трансторакальной ЭхоКГ для подтверждения/исключения тромба в полости ЛЖ рекомендуется рассмотреть возможность выполнения контрастной ЭхоКГ — при наличии физических и технических возможностей [497].
ЕОК IIbC (УУР С, УДД 4)
Комментарии. Метод контрастной ЭхоКГ в настоящее время в РФ на стадии внедрения в клиническую практику.
- При наличии тромба в полости ЛЖ у пациентов с ИМбпST рекомендуется назначение оральных антикоагулянтов (антагонистов витамина К или прямых оральных антикоагулянтов (апиксабана**, дабигатрана этексилата**, ривароксабана**, эдоксабана)) на 3-6 мес. [3][498].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. Абсолютным показанием для назначения антикоагулянтов являются следующие особенности тромба: мобильный (флотирующий) свободный участок, большие размеры (>2-3 см) и выраженная протрузия (выпячивание) тромба в полость ЛЖ, неоднородность структуры, фрагментация тромба.
Традиционной терапией внутриполостного тромба ЛЖ является терапия антагонистом витамина К под контролем МНО (целевые значения — 2,0-3,0), в период подбора дозы антагониста витамина К следует продолжать введение лечебной дозы парентерального антикоагулянта. Использование прямых оральных антикоагулянтов не исключено, но пока мало изучено.
3.4.6. Ранняя постинфарктная стенокардия и рецидив ИМ
Ранняя постинфарктная стенокардия является вариантом НС и требует неотложных лечебных мероприятий. В основе ранней постинфарктной стенокардии может лежать как ретромбоз (частичный или полный) инфаркт-связанной артерии, так и стенозирующее поражение других КА. После выполнения ЧКВ причиной возобновления ангинозных приступов может быть тромбоз стента или диссекция КА. В ранние сроки после ИМ может отмечаться появление стенокардитических болей, обусловленных ишемией жизнеспособного миокарда в периинфарктной зоне (особенно если не проводилась реперфузионная терапия) или, реже, в других сосудистых бассейнах. Ишемия может быть связана с еще одной АСБ, иногда расположенной в другой КА.
Следует дифференцировать раннюю постинфарктную стенокардию от болей, связанных с перикардитом.
В раннем периоде (до 28 дней включительно) ИМпST может развиться рецидив ИМ, приводящий к расширению зоны поражения. Рецидив ИМ следует исключать при повторном ангинозном приступе длительностью >20 мин, сопровождающемся повышением уровня кардиомаркеров, изменениями ЭКГ и появлением новых зон нарушенной локальной сократимости.
- У пациентов с ИМбпST при возникновении ангинозного болевого приступа длительностью >20 мин для диагностики рецидива ИМ рекомендуется исследование уровня сердечного тропонина I или Т с повторной оценкой через 6 ч. Повышение уровня сердечного тропонина на ≥20% от предшествующего уровня в сочетании с критериями ишемии миокарда свидетельствует о рецидиве ИМ [5].
ЕОК IIaC (УУР С, УДД 5)
- Для предотвращения распространения зоны ИМ у пациентов с ИМбпST и ранней постинфарктной стенокардией и/или рецидивом ИМ рекомендуется проведение КГ и, при необходимости, выполнение реваскуляризации [3][149].
ЕОК IIaC (УУР С, УДД 5)
3.5. Инфаркт миокарда без стойкого подъема сегмента ST правого желудочка
Изолированный ИМ ПЖ встречается редко, чаще он отмечается в сочетании с ИМ нижней стенки ЛЖ. Клинически для ИМ ПЖ характерны артериальная гипотензия, набухание вен шеи при отсутствии признаков застоя в малом круге. Гипотония при ИМ ПЖ связана с относительной гиповолемией.
- У пациентов с ИМбпST нижней локализации для исключения/выявления вовлечения в зону некроза миокарда ПЖ рекомендуется регистрировать ЭКГ в правых прекордиальных отведениях (элевация сегмента ST ≥1 мм в отведениях V3R и V4R является признаком ИМ ПЖ) и выполнить ЭхоКГ с целью поиска нарушений локальной сократимости ПЖ, расширения его полости [3][68].
ЕОК IIaC (УУР С, УДД 5)
Комментарии. ЭхоКГ позволяет выявить нарушения локальной сократимости ПЖ, расширение его полости.
- У пациентов с ИМбпST с вовлечением ПЖ для устранения гипотонии рекомендуется обеспечить увеличение объема притекающей к правым отделам сердца крови с помощью введения плазмоэкспандеров (например, раствор натрия хлорида** 0,9%). В более тяжелой ситуации рекомендуются адрено- и допаминстимуляторы (группа C01CA — адренергические и дофаминергические средства) [3].
ЕОК IIaC (УУР С, УДД 5)
- При ИМбпST c вовлечением ПЖ из-за угрозы усугубления относительной гиповолемии рекомендуется избегать назначения диуретиков и особенно периферических вазодилататоров [3, 12].
ЕОК IIIC (УУР С, УДД 5)
3.6. Лечение пациентов пожилого и старческого возраста
- У пациентов пожилого и старческого возраста с ОКСбпST рекомендуется применять те же стратегии диагностики и лечения, что и у пациентов более молодого возраста — для улучшения их клинического состояния и прогноза [499][500].
ЕОК IВ (УУР А, УДД 2)
Комментарии. Противопоказанием или ограничением к применению того или иного метода могут стать нарушения функции органов и систем органов, но не возраст сам по себе.
Анатомическая сложность поражения КА у пациентов пожилого и старческого возраста, наличие возраст-ассоциированных заболеваний и гериатрических синдромов (старческой астении, падений, когнитивных нарушений, недостаточности питания, полипрагмазии, снижения физического функционирования, инструментальной и базовой функциональной активности и др.) могут снизить эффективность лечения ОКСбпST и ухудшить прогноз таких пациентов как в период госпитализации, так и после выписки [401][501-504].
- С целью повышения безопасности применения антитромботических средств и препаратов вторичной профилактики у пациентов пожилого и старческого возраста с ОКСбпST выбор и дозы этих лекарственных средств рекомендуется адаптировать с учетом фильтрационной функции почек, сопутствующих заболеваний, наличия гериатрических синдромов (старческой астении, падений, когнитивных нарушений, полипрагмазии и др.) и конкретных противопоказаний к лекарственным средствам [499][500][505][506].
ЕОК IВ (УУР А, УДД 2)
Комментарии. У пациентов пожилого и старческого возраста с ИМпST в период госпитализации целесообразно проводить оценку наличия гериатрических синдромов и осуществление профилактических мероприятий для снижения риска развития делирия, недостаточности питания, падений, а также планирование выписки, амбулаторного наблюдения, реабилитационных мероприятий с учетом функционального статуса и потребности в социальной помощи/системе долговременного ухода.
В идеале ведение пациентов пожилого и старческого возраста с ОКСбпST должно осуществляться мультидисциплинарной командой с участием врача-гериатра или врача-кардиолога, имеющего подготовку по гериатрии, начиная с момента нахождения в блоке интенсивной терапии [507][508].
Скрининг, профилактика и коррекция гериатрических синдромов у пациентов пожилого и старческого возраста с ОКСбпST осуществляются в соответствии с принципами, изложенными в клинических рекомендациях "Старческая астения", "Недостаточность питания (мальнутриция) у пациентов пожилого и старческого возраста" и других в зависимости от выявленных гериатрических синдромов7.
- С целью повышения безопасности лечения у каждого пациента с ОКСбпST с синдромом старческой астении и значимыми сопутствующими заболеваниями рекомендуется индивидуальный подход к выбору и объему интервенционного и фармакологического лечения с тщательной комплексной оценкой всех рисков и преимуществ [499][500][509-511].
ЕОК IВ (УУР А, УДД 2)
4. Медицинская реабилитация и санаторно-курортное лечение, медицинские показания и противопоказания к применению методов медицинской реабилитации, в том числе основанных на использовании природных лечебных факторов
- Всем пациентам, перенесшим ОКСбпST, рекомендуется проведение кардиологической реабилитации (КР) для снижения риска смерти и частоты госпитализаций, улучшения прогноза, качества жизни и психосоциального статуса, повышения физической работоспособности, восстановления функций сердечно-сосудистой системы, оздоровления образа жизни, коррекции сердечно-сосудистых факторов риска и повышения приверженности лечению [512-516].
ЕОК IА (УУР А, УДД 1)
Комментарии. КР представлена трехэтапной системой, в рамках которой осуществляется маршрутизация пациента: 1-й этап — в структурных подразделениях медицинской организации, оказывающих специализированную, в т. ч. высокотехнологичную, медицинскую помощь в стационарных условиях (отделении реанимации и интенсивной терапии и кардиологическом отделении, палате сосудистого центра или первичного сосудистого отделения); 2-й этап — в стационарном отделении реабилитации; 3-й этап — в амбулаторных условиях8.
Ключевыми компонентами комплексной программы КР являются: физические тренировки (контролируемые, домашние), образовательная программа (информирование и обучение в "Школе для пациентов, перенесших ОКС"), модификация основных факторов риска (курения, нездорового питания, дислипидемии, АГ, избыточной массы тела/ожирения, гиподинамии, гипергликемии при СД), психологическая поддержка, мероприятия по повышению приверженности пациентов, сочетающиеся с назначением медикаментозной терапии в соответствии с клиническими рекомендациями.
Программа КР должна начинаться как можно раньше после стабилизации клинического состояния пациента, проводиться в плановом порядке, основываться на пациент-ориентированном подходе с учетом медицинских показаний и противопоказаний к проведению отдельных компонентов программы. Всех амбулаторных пациентов, перенесших ОКС и ранее не участвовавших в программах КР, рекомендуется направлять на КР независимо от сроков заболевания9.
Исключение составляют пациенты с установленным диагнозом деменции.
Рекомендуемая общая продолжительность кардиореабилитационной программы на трех этапах составляет не менее 24 нед. Большая длительность программы КР (на амбулаторном этапе) повышает ее эффективность.
- У всех пациентов, перенесших ОКСбпST, амбулаторный этап КР рекомендуется проводить в очном, дистанционном (в домашних условиях с использованием инструментов цифрового и мобильного здравоохранения) или смешанном форматах для увеличения охвата пациентов реабилитацией и повышения их приверженности КР и лечению в целом10 [517-519][520].
ЕОК IIbВ (УУР С, УДД 2)
- Для реализации программы КР у пациентов, перенесших ОКСбпST, рекомендуется формировать мультидисциплинарную реабилитационную команду на всех этапах с целью оценки клинико-функционального состояния пациента, определения и проведения комплекса реабилитационных мероприятий11 [512][521][522].
ЕОК IA (УУР А, УДД 1)
Комментарии. В состав мультидисциплинарной реабилитационной команды должны входить: врач-кардиолог, врач по физической и реабилитационной медицине, специалист по физической реабилитации (инструктор-методист по лечебной физической культуре), медицинский психолог и/или врач-психотерапевт, врач-диетолог, медицинская сестра по медицинской реабилитации (инструктор по лечебной физической культуре) и другие специалисты по требованию. Для оценки клинического статуса пациента, уровня его функционирования и жизнедеятельности, влияния личностных факторов и факторов окружающей среды рекомендуется использовать стандартизованные и валидные методы диагностики, а также инструменты оценки по Международной Классификации Функционирования, ограничений жизнедеятельности и здоровья (МКФ) [523][524].
- Всем пациентам после ОКСбпST рекомендуется проведение теста с дозированной физической нагрузкой (теста с физической нагрузкой с использованием эргометра или тредмила) или кардиопульмонального тестирования (количественной оценки потребления кислорода в условиях эргоспирометрии) — при наличии возможности и достаточном опыте его проведения в учреждении, а при их недоступности (или противопоказаниях) — теста с 6-минутной ходьбой с целью оценки функционального статуса, толерантности к физической нагрузке, выбора оптимального режима физических тренировок, контроля их эффективности и безопасности [525][526].
ЕОК IА (УРР С, УДД 5)
Комментарии. Нагрузочное тестирование проводится перед началом программы физических тренировок и после завершения этапа КР.
- Всем пациентам, перенесшим ОКСбпST, рекомендуются аэробные физические тренировки (лечебная физкультура с использованием тренажеров при заболеваниях сердца и перикарда; индивидуальные занятия лечебной физкультурой при заболеваниях сердца и перикарда; групповые занятия лечебной физкультурой при заболеваниях сердца и перикарда; лечебная физкультура с биологической обратной связью при заболеваниях сердца и перикарда) умеренной интенсивности с целью улучшения функционального статуса, уменьшения симптоматики, повышения физической работоспособности, улучшения качества жизни, снижения риска сердечно-сосудистых осложнений, частоты госпитализаций и смертности [512][516][522][527-530].
ЕОК IА (УУР А, УДД 1)
Комментарии. У пациентов, перенесших ОКСбпST, рекомендуется перед назначением физических тренировок исключить противопоказания к ним (Приложение А3. Основные противопоказания к проведению физических тренировок) [502][531]. На раннем 1-ом этапе КР рекомендуется поэтапное расширение двигательной активности и использование комплексов физических упражнений. На более поздних (2-ом и 3-ем) этапах в программу КР рекомендуется включать физические тренировки в индивидуальном формате и/или в организованных группах в лечебном учреждении и/или в домашних условиях после обучения [532].
Рекомендуемая длительность физической тренировки динамического характера (например, на велотренажере или тредмиле) составляет не менее 30 мин (общая продолжительность тренировочного занятия 45-60 мин) при частоте занятий не менее 3 раз в нед. (оптимально — 5-6 раз в нед.); интенсивность тренирующей нагрузки умеренная (55-69% от максимальной ЧСС при нагрузочном тестировании или в зоне 12-14/20 баллов по шкале Борга; Borg Rating of Perceived Exertion, Borg RPE), уровень тренирующей нагрузки в течение всей основной фазы тренировки постоянный [512][528][531][533][534].
Для очень детренированных пациентов и пациентов с СН на старте программы могут рекомендоваться интервальные тренировки низкой интенсивности.
Для безопасности физической реабилитации рекомендуется осуществлять мониторинг состояния пациента (жалоб, клинических симптомов, уровня АД, ЧСС и ЭКГ), оценивать уровень физического напряжения по шкале Борга в процессе тренировки [534].
Пациентам, перенесшим ОКСбпST, после окончания программ КР рекомендуется поддерживать физическую активность на уровне умеренной интенсивности не менее 150 мин в нед. или высокой интенсивности не менее 75 мин в нед. Если больной не способен поддерживать рекомендуемый уровень повседневной физической активности, то этот уровень должен находиться в пределах физических возможностей пациента и соответствовать его клиническому состоянию.
- У отдельных пациентов, перенесших ОКСбпST, с низким риском осложнений (включая атеротромботический и ишемический риски) и при их желании рекомендуется рассмотреть проведение высокоинтенсивных интервальных тренировок с целью повышения физической работоспособности и улучшения качества жизни [535-537].
ЕОК IIbС (УУР А, УДД 1)
Комментарии. Назначение высокоинтенсивных интервальных тренировок рекомендуется рассматривать только на 3-ем этапе КР при отсутствии противопоказаний, после оценки всех возможных рисков и безопасности данного физического воздействия.
- Пациентам, перенесшим ОКСбпST, рекомендуется проводить оценку психосоциального статуса посредством стандартизованных опросников или клинического интервью с целью идентификации возможных барьеров для изменения образа жизни и приверженности лечению, индивидуализации программы КР и повышения ее эффективности, оказания своевременной психологической поддержки, улучшения качества жизни и снижения сердечно-сосудистых осложнений [538-540].
ЕОК IВ (УУР С, УДД 5)
Комментарии. При наличии клинически выраженной тревожной и/или депрессивной симптоматики рекомендуется психологическое консультирование и поддержка, назначение психофармакотерапии (по показаниям), что в сочетании со структурированным обучением, физическими тренировками может уменьшать симптомы депрессии, улучшать социальное функционирование, когнитивный профиль и качество жизни пациента [539][541-544].
- Всем пациентам, перенесшим ОКСбпST, рекомендуется проведение образовательной программы в любом доступном формате (индивидуально и/или в группах — "Школе для пациентов, перенесших ОКС" в очном или онлайн режимах) с целью повышения их информированности о заболевании, его факторах риска и профилактики; оздоровления образа жизни, обучения методам самоконтроля и самопомощи, повышения приверженности лечебно-реабилитационным вмешательствам [545][546].
ЕОК IA (УУР С, УДД 5)
Комментарии. Рекомендуется провести не менее 6-10 занятий; при увеличении количества занятий эффективность обучающей программы повышается (эти занятия могут проводиться в формате дистанционной поддержки).
5. Профилактика и диспансерное наблюдение, медицинские показания и противопоказания к применению методов профилактики
5.1. Профилактика
Помимо продолжения медикаментозной терапии, начатой в ранние сроки лечения ОКСбпST (Раздел 3.1), рекомендуются вмешательства по контролю сердечно-сосудистых факторов риска и предупреждению внезапной сердечной смерти (Раздел 3.3.2.1).
- Для снижения риска сердечно-сосудистых событий среди пациентов, перенесших ОКСбпST, рекомендуются выявление курильщиков и регулярные вмешательства, направленные на полный отказ от курения, включая отказ от пассивного курения [547-560].
ЕОК IIaA (УУР A, УДД 1)
Комментарии. Мероприятия по отказу от курения рекомендуется начинать на госпитальном этапе и продолжить после выписки. Рекомендуются поведенческие и фармакологические вмешательства (никотинзаместительная терапия, варениклин или бупропион).
- Для снижения риска неблагоприятных исходов пациентам, перенесшим ОКСбпST, рекомендуется придерживаться принципов здорового питания, поддерживать нормальную массу тела, выполнять регулярные физические упражнения и ограничить прием алкоголя [3][512][561-576].
ЕОК IВ (УУР B, УДД 2)
Комментарии. Ключевые характеристики здорового питания: ограничение потребления насыщенных жиров и транс-изомеров жирных кислот до <10% и <1% от общей калорийности суточного рациона, соответственно; поваренной соли до 5 г в сут.; легкоусваиваемых углеводов; потребление большого количества фруктов (≥250 г в сут.), овощей (≥250 г в сут.) и цельнозерновых продуктов. Рекомендуется потребление рыбы 1-2 раза в нед. (хотя бы 1 раз в нед. — жирной рыбы), а также молочных продуктов с низкой жирностью, постного мяса и птицы, бобовых и несоленых орехов в количестве 30 г в день.
Суточное потребление алкоголя не должно превышать 1 стандартную дозу у женщин и 1-2 стандартных дозы у мужчин (1 стандартная доза — 40 мл крепких спиртных напитков или 120 мл сухого вина или 330 мл пива).
Целевые значения индекса массы тела составляют 20-25 кг/м², окружности талии — <80 см для женщин и <94 см для мужчин.
- У пациентов, перенесших ОКСбпST, для снижения риска ишемических событий и смерти рекомендуется контроль АД и поддержание его на целевом уровне [367][375][577-580].
ЕОК IA (УУР A, УДД 1)
Комментарии. Целевой уровень АД у пациентов после ОКСбпST — <140/90 мм рт.ст. При хорошей переносимости следует рассмотреть снижение АД до 130/80 мм рт.ст. и ниже. Для контроля АД после ОКСбпST предпочтительны β-АБ и иАПФ.
- У пациентов с СД, перенесших ОКСбпST, рекомендуется проводить лечение, направленное на поддержание выбранного совместно с врачом-эндокринологом индивидуального целевого уровня гликированного гемоглобина (у большинства пациентов — <7,0%) для снижения риска микро- и макрососудистых осложнений СД [581-584].
ЕОК IIaB (УУР В, УДД 2)
Комментарии. У пациентов c СД 2 типа перспективно использование аналогов глюкагоноподобного пептида-1 (GLP-1) и ингибиторов натрийзависимого переносчика глюкозы 2 типа, способствующих снижению частоты сердечно-сосудистых осложнений у пациентов с атеросклеротическими сердечно-сосудистыми заболеваниями.
- Пациентам, перенесшим ОКСбпST, для снижения риска сердечно-сосудистых осложнений и смерти рекомендуется ежегодная вакцинация против гриппа [585-593].
ЕОК IА (УУР А, УДД 2)
- При недостаточном контроле факторов риска или возникновении сердечно-сосудистых осложнений на фоне оптимальной медикаментозной терапии у пациентов, перенесших ОКСбпST, рекомендуется рассмотреть возможность применения низких доз (0,5 мг/день) #безвременника осеннего семян экстракта (колхицина) [594][595].
ЕОК IIbA (УУР А, УДД 2)
- У пациентов, перенесших ОКСбпST, рекомендуется использовать фиксированные комбинации препаратов для увеличения приверженности к лечению и уменьшения риска неблагоприятных исходов [596-598].
ЕОК IIaB (УУР B, УДД 2)
5.2. Диспансерное наблюдение
- При выписке из стационара пациента, перенесшего ОКСбпST (ИМбпST или НС), ему необходимо дать информацию о его состоянии в устной и письменной форме [599][600].
ЕОК IIaB (УУР В, УДД 3)
- Рекомендуется диспансерное наблюдение за всеми пациентами, перенесшими ОКСбпST (ИМбпST или НС), после их выписки из стационара для реализации комплекса мер по профилактике и лечению сердечно-сосудистых осложнений, своевременной коррекции терапии и повышения приверженности к лечению12, 13 [3].
ЕОК IC (УУР C, УДД 5)
- Рекомендуется, чтобы диспансерное наблюдение за всеми пациентами в первые 12 мес. после ОКСбпST (ИМбпST или НС) осуществлялось врачом-кардиологом для реализации комплекса мер по профилактике и лечению сердечно-сосудистых осложнений, своевременной коррекции терапии и повышения приверженности к лечению. Минимальная периодичность диспансерных приемов составляет 2 раза в год; при осложненном течении заболевания, а также при необходимости титрования доз лекарственных средств (гиполипидемических, β-АБ, блокаторов ренин-ангиотензин-альдостероновой системы, антагонистов витамина К и др.) частоту диспансерных приемов рекомендуется определять в соответствии с клинической необходимостью13.
ЕОК IC (УУР C, УДД 5)
Комментарии. В соответствии с Приказом Министерства здравоохранения Российской Федерации от 15 марта 2022г № 168н "Об утверждении порядка проведения диспансерного наблюдения за взрослыми", диспансерное наблюдение пациентов в первые 12 мес. после ИМ, ЧКВ или КШ осуществляется врачом-кардиологом. Пациенты с НС в данном Приказе не упомянуты. Однако в связи с неблагоприятным прогнозом после эпизода НС, такие больные тоже нуждаются в диспансерном наблюдении врачом-кардиологом, даже если им не были выполнены ЧКВ или КШ.
В соответствии с Приказом Министерства здравоохранения Российской Федерации от 15 марта 2022г № 168н "Об утверждении порядка проведения диспансерного наблюдения за взрослыми" спустя 12 мес. пожизненное диспансерное наблюдение осуществляется врачом-терапевтом участковым (семейным врачом), прием (осмотр, консультация) врача-кардиолога осуществляется по медицинским показаниям при направлении врача-терапевта участкового (семейного врача).
Продолжение диспансерного наблюдения врачом-кардиологом предписывается при стенокардии напряжения III-IV ФК, неэффективности медикаментозной терапии (рефрактерные симптомы, недостижение целевых уровней АД, ЧСС, ХС ЛНП), наличии СН, жизнеугрожающих нарушений ритма, СД, ХБП С4 и C5 стадий, комбинированной антитромботической терапии, симптомном заболевании периферических артерий, атеросклерозе другого сосудистого бассейна при назначении двойной антиагрегантной или комбинированной антитромботической терапии.
На визитах рекомендуется измерять АД, ЧСС, определять массу тела (с расчетом индекса массы тела), измерять окружность талии, оценивать статус курения13.
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), для оценки нарушений липидного обмена и контроля эффективности гиполипидемической терапии рекомендуется выполнять анализ крови для оценки нарушений липидного обмена биохимический (как минимум исследование уровня ХС ЛНП) с периодичностью не реже 2 раз в год13.
ЕОК IC (УУР C, УДД 5)
Комментарии. При подборе гиполипидемической терапии уровень ХС ЛНП рекомендуется оценивать каждые 4-6 нед., пока не будут достигнуты целевые значения показателя (<1,4 ммоль/л со снижения как минимум на 50% от исходных значений).
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), рекомендуется выполнять общий (клинический) анализ крови и биохимический общетерапевтический анализы крови (с расчетом рСКФ) с периодичностью не реже 1 раза в год для контроля безопасности лечения и своевременной коррекции терапии13.
ЕОК IC (УУР C, УДД 5)
Комментарии. Общий (клинический) и/или биохимический общетерапевтический анализы крови могут выполняться чаще в соответствии с клинической необходимостью.
При применении прямых оральных антикоагулянтов рекомендуется определение клиренса креатинина.
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), принимающих антагонисты витамина К, рекомендуется определение МНО с периодичностью не реже 2 раз в год, для контроля эффективности и безопасности лечения, а также подбора доза препарата13.
ЕОК IC (УУР C, УДД 5)
Комментарии. У большинства пациентов, принимающих антагонисты витамина К, рекомендуется определение МНО 1 раз в мес.; в период подбора дозы антагонистов витамина К или при меняющихся значениях МНО возможны более частые определения МНО.
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), рекомендуется регистрация ЭКГ с периодичностью не реже 1 раза в год для выявления признаков прогрессирования заболевания, нарушений сердечного ритма и проводимости, а также своевременной коррекции терапии13.
ЕОК IC (УУР C, УДД 5)
Комментарии. ЭКГ может регистрироваться чаще в соответствии с клинической необходимостью.
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), рекомендуется обзорная рентгенография органов грудной клетки с периодичностью не реже 1 раза в год для выявления признаков застоя в малом круге кровообращения13.
ЕОК IC (УУР C, УДД 5)
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), рекомендуется ЭхоКГ с обязательной оценкой ФВ ЛЖ с периодичностью не реже 1 раза в год для контроля динамики изменений, связанных с перенесенным ОКС, выявления новых патологических изменений и своевременной коррекции терапии13.
ЕОК IC (УУР C, УДД 5)
Комментарии. ЭхоКГ может выполняться чаще в соответствии с клинической необходимостью.
- У пациентов, перенесших ОКСбпST (ИМбпST или НС), перенесших ЧКВ или КШ, рекомендуется выполнение неинвазивного стресс-теста (см. Термины и определения) с периодичностью не реже 1 раза в 2 года для выявления ишемии миокарда и/или оценки ее выраженности13.
ЕОК IC (УУР C, УДД 5)
Комментарии. Неинвазивные стресс-тесты могут выполняться у всех пациентов, перенесших ОКСбпST (ИМбпST или НС), в соответствии с клинической необходимостью.
6. Организация оказания медицинской помощи
6.1. Показания для госпитализации
Любое подозрение на ОКСбпST является показанием для экстренной госпитализации.
Пациенты ОКСбпST должны госпитализироваться в региональные сосудистые центры для пациентов с ОКС, в кардиологические отделения с палатой реанимации и интенсивной терапии для пациентов с ОКС (первичные сосудистые отделения). Поскольку многим пациентам с подозрением на ОКС может потребоваться углубленная дифференциальная диагностика, их оптимально госпитализировать в многопрофильный стационар с возможностью экстренной диагностики и лечения острой коронарной и иной патологии. Маршрутизация пациентов должна быть организована так, чтобы все они госпитализировались или как можно быстрее переводились в стационар с возможностью инвазивного лечения ОКС.
- На догоспитальном этапе не рекомендуется проведение инструментальных/лабораторных диагностических мероприятий, за исключением ЭКГ, направленных на подтверждение или исключение ОКСбпST (ИМбпST или НС) [3][601-606].
ЕОК IC (УУР С, УДД 5)
Комментарии. На догоспитальном этапе для принятия решения о дальнейшем ведении пациента с подозрением на ОКСбпST достаточно регистрации ЭКГ. Определять уровень маркеров повреждения миокарда нецелесообразно. В случае оказания помощи на догоспитальном этапе фельдшерской бригадой обязательна передача ЭКГ по каналам связи в специализированный телемедицинский центр с целью согласования ведения и маршрутизации пациента.
- При подозрении на ОКСбпST пациента, госпитализированного в стационар, не располагающий возможностями для экстренной реваскуляризации, рекомендуется своевременно перевести в лечебное учреждение, где реализуется программа реваскуляризации при ОКС [3].
ЕОК IC (УУР С, УДД 5)
- В каждом регионе рекомендуется разработать региональный протокол маршрутизации пациентов с ОКСбпST с учетом действующих рекомендаций и особенностей региона [3].
ЕОК IC (УУР С, УДД 5)
- В каждом регионе рекомендуется выделить специализированное кардиохирургическое отделение для проведения экстренных операций КШ у пациентов с ОКС, а также разработать региональные правила экстренного перевода таких пациентов [3].
ЕОК IC (УУР С, УДД 5)
6.2. Показания к выписке пациента из стационара
Продолжительность госпитализации определяется индивидуально в соответствии с сердечным риском пациента, сопутствующими заболеваниями, функциональным статусом и социальной поддержкой. В то же время пациенты не должны находиться в больнице дольше необходимого по психосоциальным причинам и с целью предупреждения внутригоспитальных инфекций.
После исключения диагноза ОКСбпST (например, после серийного определения динамики уровня тропонинов крови, возможно дополненного другими инструментальными методами) пациента можно перевести в профильное отделение (при наличии показаний для госпитализации) или выписать.
Пациентов с ОКСбпST рекомендуется выписывать при условии клинической стабилизации, завершения стратификации риска неблагоприятного исхода (включая КГ и/или неинвазивные стресс-тесты в случаях, когда это показано) и реализации выбранной стратегии лечения. При подтверждении предварительного диагноза ОКСбпST должен быть сформулирован окончательный клинический диагноз — ИМбпST или НС.
Безопасность ранней выписки (48-96 ч) после ЧКВ определяется в первую очередь отсутствием ранних осложнений, таких как рецидив ишемии, тромбоз стента, ОСН, наджелудочковые и ЖА, нарушения проводимости, разрыв МЖП или свободной стенки ЛЖ, острая митральная недостаточность, перикардит, тромб ЛЖ с угрозой системной эмболии.
Выполненное ЧКВ и агрессивная антитромботическая терапия могут служить причиной развития кровотечений, как связанных с местом доступа, так и других, в первую очередь желудочно-кишечных и церебральных, а также контраст-индуцированного острого почечного повреждения. Рецидив ИМ в послеоперационном периоде может быть связан либо с тромбозом стента, либо с критическим стенозом или разрывом нестабильной бляшки в изначально не симптом-связанном сегменте КА.
- Ранняя выписка пациентов с ОКСбпST после ЧКВ рекомендована у отдельных пациентов, если организованы ранняя реабилитация и адекватное наблюдение [273][288][513].
ЕОК IB (УУР A, УДД 2)
Комментарии. В случае ранней выписки за время госпитализации пациент должен начать реабилитацию, получить подробную информацию о случившемся, а также о предстоящей модификации образа жизни. Кроме того, должна быть инициирована медикаментозная терапия в рамках вторичной профилактики (оптимальная антитромбоцитарная терапия, β-АБ, блокатор(-ы) ренин-ангиотензин-альдостероновой системы, ингибитор ГМГ-КоА-редуктазы).
6.3. Иные организационные технологии
При подозрении на ОКСбпST рекомендуется интенсивное наблюдение за пациентами в блоке (палате) интенсивной терапии с дистанционным наблюдением за электрокардиографическими данными (мониторированием ЭКГ) для контроля ритма сердца, пока не будет подтверждено или отвергнуто наличие ОКСбпST, определен риск неблагоприятного исхода, выбрана стратегия ведения пациента и исключены другие угрожающие жизни заболевания и осложнения.
При неосложненном ИМбпST у пациентов, не имеющих признаков, указывающих на повышенный риск возникновения аритмий, рекомендуется интенсивное наблюдение в блоке (палате) интенсивной терапии с дистанционным наблюдением за электрокардиографическими данными (мониторированием ЭКГ) для контроля ритма сердца продолжительностью до 24 ч или до успешного ЧКВ, если оно было выполнено в первые сутки после госпитализации. В остальных случаях интенсивное наблюдение с дистанционным наблюдением за электрокардиографическими данными (мониторированием ЭКГ) для контроля ритма сердца рекомендуется продлить до клинической стабилизации.
Дистанционное наблюдение за электрокардиографическими данными (мониторированием ЭКГ) для контроля ритма сердца рекомендуется продлить при повышенном риске возникновения аритмий (нестабильность гемодинамики, имевшиеся серьезные аритмии, ФВ ЛЖ ≤40%, безуспешные попытки реперфузионного лечения, сохраняющиеся критические стенозы в крупных КА, осложнения при ЧКВ, сумма баллов по шкале GRACE >140). У пациентов, не имеющих признаков продолжающейся ишемии миокарда, продление дистанционного наблюдения за электрокардиографическими данными (мониторирования ЭКГ) для контроля ритма сердца возможно при подозрении на спазм КА, а также при наличии симптомов, не позволяющих исключить аритмию.
7. Дополнительная информация (в том числе факторы, влияющие на исход заболевания или состояния)
Дополнительная информация представлена в Приложениях А3 и Б1-Б3.
Критерии оценки качества медицинской помощи
Критерии оценки качества первичной медико-санитарной помощи взрослым при ОКСбпST электрокардиограммы (коды по МКБ-10: I20.0, I21.0, I21.1, I21.2, I21.3, I21.4, I21.9, I22.0, I22.1, I22.8, I22.9, I24.8, I24.9)
|
N п/п |
Критерии оценки качества |
Оценка выполнения |
|
1. |
После выписки из стационара пациент взят на диспансерное наблюдение |
Да/Нет |
|
2. |
Осуществляется программа кардиологической реабилитации |
Да/Нет |
|
3. |
Выполнен общий (клинический) анализ крови с периодичностью не реже 1 раза в год |
Да/Нет |
|
4. |
Выполнен биохимический общетерапевтический анализ крови (с расчетом рСКФ) с периодичностью не реже 1 раза в год |
Да/Нет |
|
5. |
Выполнен анализ крови по оценке нарушений липидного обмена биохимический (как минимум исследование ХС ЛНП) с периодичностью не реже 2 раз в год |
Да/Нет |
|
6. |
Выполнена регистрация ЭКГ с периодичностью не реже 1 раза в год |
Да/Нет |
|
7. |
Выполнена ЭхоКГ с оценкой ФВ ЛЖ с периодичностью не реже 1 раза в год |
Да/Нет |
|
8. |
Проводится терапия АСК в сочетании с ингибитором Р2Y12-рецепторов тромбоцитов (клопидогрел, тикагрелор или прасугрел), или терапия пероральным антикоагулянтом (апиксабан, дабигатрана этексилат, ривароксабан, эдоксабан или антагонист витамина К) в сочетании с антиагрегантом (клопидогрелом, реже тикагрелором) в зависимости от медицинских показаний и при отсутствии медицинских противопоказаний |
Да/Нет |
|
9. |
Проводится терапия высокими дозами лекарственных препаратов из группы ингибиторов ГМГ-КоА-редуктазы при отсутствии медицинских противопоказаний; при недостижении целевого уровня ХС ЛНП (<1,4 ммоль/л) и его снижения на 50% от исходного увеличена доза ингибитора ГМГ-КоА-редуктазы или проводится комбинированная терапия (с эзетимибом и/или препаратом из группы другие гиполипидемические средства (ингибиторы PCSK9)) в зависимости от медицинских показаний и при отсутствии медицинских противопоказаний |
Да/Нет |
|
10. |
Проводится терапия лекарственными препаратами из группы иАПФ или АРА (как минимум у пациентов с ФВ ЛЖ ≤40%, сердечной недостаточностью, артериальной гипертензией, хронической болезнью почек, при сахарном диабете) в зависимости от медицинских показаний и при отсутствии медицинских противопоказаний |
Да/Нет |
|
11. |
Проводится терапия лекарственными препаратом из группы бета-адреноблокаторов перорально (как минимум при ФВ ЛЖ ≤40%) при отсутствии медицинских противопоказаний |
Да/Нет |
Сокращения: АРА — антагонисты рецепторов ангиотензина II, АСК — ацетилсалициловая кислота, ГМГ-КоА редуктаза — 3-гидрокси-3-метилглутарил коэнзима А редуктаза, иАПФ — ингибиторы ангиотензинпревращающего фермента, ЛЖ — левый желудочек, ЛНП — липопротеиды низкой плотности, рСКФ — расчётная скорость клубочковой фильтрации, ФВ — фракция выброса, ХС — холестерин, ЭКГ — электрокардиография (син.: регистрация электрокардиограммы), ЭхоКГ — эхокардиография.
Критерии оценки качества специализированной медицинской помощи взрослым при ОКСбпST электрокардиограммы (коды по МКБ-10: I20.0, I21.0, I21.1, I21.2, I21.3, I21.4, I21.9, I22.0, I22.1, I22.8, I22.9, I24.8, I24.9)
|
N п/п |
Критерии оценки качества |
Оценка выполнения |
|
1. |
Выполнено исследование уровня сердечного тропонина I или T в крови (при неинформативности первого исследования повторно) |
Да/Нет |
|
2. |
Выполнен ЭхоКГ с оценкой ФВ ЛЖ |
Да/Нет |
|
3. |
Проведена оценка риска неблагоприятного исхода, выбрана и реализована одна из стратегий лечения в стационаре: КГ с намерением выполнить реваскуляризацию миокарда в первые 2 ч после госпитализации, КГ с намерением выполнить реваскуляризацию миокарда в первые 24 ч после госпитализации, КГ с намерением выполнить реваскуляризацию миокарда до выписки или первоначальное неинвазивное лечение |
Да/Нет |
|
4. |
Проводится терапия лекарственными препаратами из группы иАПФ или АРА (как минимум у пациентов с ФВ ЛЖ ≤40%, сердечной недостаточностью, артериальной гипертензией, хронической болезнью почек, при сахарном диабете) в зависимости от медицинских показаний и при отсутствии медицинских противопоказаний |
Да/Нет |
|
5. |
Проводится терапия лекарственным препаратом из группы бета-адреноблокаторов перорально (как минимум при ФВ ЛЖ ≤40%) при отсутствии медицинских противопоказаний |
Да/Нет |
|
6. |
Начата гиполипидемическая терапия высокими дозами лекарственных препаратов из группы ингибиторов ГМГ-КоА-редуктазы при отсутствии медицинских противопоказаний |
Да/Нет |
|
7. |
В начале лечения использовалось сочетание АСК, ингибитора Р2Y12-рецептора тромбоцитов (клопидогрел, тикагрелор или прасугрел) и антикоагулянта с последующим переходом на сочетание АСК с ингибитором Р2Y12-рецептора тромбоцитов или, для пациентов с показаниями к длительному применению антикоагулянтов, на сочетание перорального антикоагулянта с антиагрегантом в зависимости от медицинских показаний и при отсутствии медицинских противопоказаний |
Да/Нет |
|
8. |
Перед выпиской из стационара определены сроки этапной реваскуляризации (при наличии показаний) |
Да/Нет |
Сокращения: АРА — антагонисты рецепторов ангиотензина II, АСК — ацетилсалициловая кислота, ГМГ-КоА редуктаза — 3-гидрокси-3-метилглутарил коэнзима А редуктаза, иАПФ — ингибиторы ангиотензинпревращающего фермента, КГ — коронарография, ЛЖ — левый желудочек, ФВ — фракция выброса, ЭхоКГ — эхокардиография.
1. Федеральный закон от 21.11.2011 № 323-ФЗ (ред. от 24.07.2023) "Об основах охраны здоровья граждан в Российской Федерации" (с изм. и доп., вступ. в силу с 01.09.2023).
2. Эпидемиологический словарь, 4-е издание. Под редакцией Джона М. Ласта для Международной эпидемиологической ассоциации. Москва, 2009, 316 с.
3. Федеральное агентство по техническому регулированию и метрологии. Национальный стандарт Российской Федерации. ГОСТР 52379-2005. Надлежащая клиническая практика. Москва, 2005.
4. Федеральный закон от 12.04.2010 № 61-ФЗ (ред. от 03.07.2016) "Об обращении лекарственных средств".
5. Малая медицинская энциклопедия. — М.: Медицинская энциклопедия. 1991-96 гг. [Электронный ресурс]. Режим доступа: http://dic.academic.ru/dic.nsf/enc_medicine/28878/Синдром.
6. Федеральная служба государственной статистики. Статистические данные по Российской Федерации. 2022 год. https://rosstat.gov.ru/folder/12781.
7. Клинические рекомендации Минздрава России "Старческая астения" https://cr.minzdrav.gov.ru/recomend/613_1; Клинические рекомендации Минздрава России "Недостаточность питания (мальнутриция) у пациентов пожилого и старческого возраста" https://cr.minzdrav.gov.ru/recomend/615_2.
8. Приказ Минздрава России от 31 июля 2020г № 778н "О Порядке организации медицинской реабилитации взрослых". Зарегистрировано в Минюсте РФ 25 сентября 2020г. Регистрационный № 60039 https://www.garant.ru/products/ipo/prime/doc/74581688/.
9. Приказ Минздрава России от 31 июля 2020г № 778н "О Порядке организации медицинской реабилитации взрослых". Зарегистрировано в Минюсте РФ 25 сентября 2020г. Регистрационный № 60039 https://www.garant.ru/products/ipo/prime/doc/74581688/.
10. Федеральный закон от 29.07.2017 № 242-ФЗ "О внесении изменений в отдельные законодательные акты Российской Федерации по вопросам применения информационных технологий в сфере охраны здоровья" https://www.garant.ru/products/ipo/prime/doc/716328 44/.
11. Приказ Минздрава России от 31 июля 2020г № 778н "О Порядке организации медицинской реабилитации взрослых" Зарегистрировано в Минюсте РФ 25 сентября 2020г. Регистрационный № 60039 https://www.garant.ru/products/ipo/prime/doc/74581688/.
12. Приказ Минздрава России от 15 ноября 2012г № 918н "Об утверждении Порядка оказания медицинской помощи больным с сердечно-сосудистыми заболеваниями" (с изменениями и дополнениями от 21.02.2020г).
13. Приказ Минздрава России от 15 марта 2022г № 168н "Об утверждении порядка проведения диспансерного наблюдения за взрослыми".
Приложение А1. Состав Рабочей группы по разработке и пересмотру клинических рекомендаций
Президиум Рабочей группы:
Аверков О. В. (Москва)
Арутюнян Г. К. (Москва)
Дупляков Д. В. (Самара)
Константинова Е. В. (Москва)
Никулина Н. Н. (Рязань)
Шахнович Р. М. (Москва)
Явелов И. С. (Москва)
Яковлев А. Н. (Санкт-Петербург)
Другие члены Рабочей группы:
Абугов С. А. (Москва)
Алекян Б. Г. (Москва)
Аронов Д. М. (Москва)
Архипов М. В. (Екатеринбург)
Барбараш О. Л. (Кемерово)
Бойцов С. А. (Москва)
Бубнова М. Г. (Москва)
Вавилова Т. В. (Санкт-Петербург)
Васильева Е. Ю. (Москва)
Галявич А. С. (Казань)
Ганюков В. И. (Кемерово)
Гиляревский С. Р. (Москва)
Голубев Е. П. (Москва)
Голухова Е. З. (Москва)
Затейщиков Д. А. (Москва)
Карпов Ю. А. (Москва)
Космачева Е. Д. (Краснодар)
Лопатин Ю. М. (Волгоград)
Марков В. А. (Томск)
Меркулов Е. В. (Москва)
Новикова Н. А. (Москва)
Панченко Е. П. (Москва)
Певзнер Д. В. (Москва)
Погосова Н. В. (Москва)
Прасол Д. М. (Санкт-Петербург)
Протопопов А. В. (Красноярск)
Скрыпник Д. В. (Москва)
Тарасов Р. С. (Москва)
Терещенко С. Н. (Москва)
Устюгов С. А. (Красноярск)
Хрипун А. В. (Ростов-на-Дону)
Цебровская Е. А. (Санкт-Петербург)
Шалаев С. В. (Тюмень)
Шляхто Е. В. (Санкт-Петербург)
Шпектор А. В. (Москва)
Якушин С. С. (Рязань)
Все члены Рабочей группы подтвердили отсутствие финансовой поддержки/конфликта интересов, о которых необходимо сообщить. В случае сообщения о наличии конфликта интересов члены Рабочей группы были исключены из обсуждения разделов, связанных с областью конфликта интересов.
Приложение А2. Методология разработки клинических рекомендаций
В основе рекомендаций лежат результаты крупных многоцентровых исследований, метаанализов, регистров, которые являются основой и для других национальных и международных клинических рекомендаций. Учтены основные положения обновленных рекомендаций по диагностике и лечению ОКС Европейского общества кардиологов (ЕОК) и Американских Коллегии кардиологов/Ассоциации сердца, рекомендаций ЕОК по реваскуляризации миокарда, рекомендации ЕОК по двойной антитромбоцитарной терапии, рекомендаций ЕОК по хроническим коронарным синдромам, а также рекомендаций указанных профессиональных сообществ по антитромботической терапии у пациентов с ФП. При этом учитывались отличия и особенности оказания медицинской помощи пациентам с ОКС в Российской Федерации.
Целевая аудитория данных клинических рекомендаций:
- Врач-кардиолог.
- Врач-анестезиолог-реаниматолог.
- Фельдшер скорой медицинской помощи.
- Врач скорой медицинской помощи.
- Врач-терапевт.
- Врач-терапевт участковый.
- Врач общей практики (семейный врач).
- Врач-сердечно-сосудистый хирург.
- Врач по рентгенэндоваскулярным методам диагностики и лечения.
Шкала оценки классов рекомендаций Европейского общества кардиологов (ЕОК)
Вследствие того, что члены Российского кардиологического общества (РКО) входят в состав Европейского общества кардиологов и также являются его членами, все рекомендации ЕОК формируются с участием российских экспертов, которые являются соавторами европейских рекомендаций. Таким образом, существующие рекомендации ЕОК отражают общее мнение ведущих российских и европейских кардиологов. В связи с этим формирование Национальных рекомендаций проводилось на основе рекомендаций ЕОК, с учетом национальной специфики, особенностей обследования, лечения, учитывающих доступность медицинской помощи. По этой причине в тексте настоящих клинических рекомендаций одновременно использованы две шкалы оценки достоверности доказательств тезисов рекомендаций: уровни достоверности доказательств ЕОК с УУР и УДД. Добавлены классы рекомендаций ЕОК, позволяющие оценить необходимость выполнения тезиса рекомендаций (табл. 1-5).
Таблица 1
Классы показаний согласно рекомендациям ЕОК
|
Класс рекомендаций ЕОК |
Определение |
Предлагаемая формулировка |
|
I |
Доказано или общепризнанно, что диагностическая процедура, вмешательство/лечение являются эффективными и полезными |
Рекомендовано/показано |
|
II IIa IIb |
Противоречивые данные и/или мнения об эффективности/пользе диагностической процедуры, вмешательства, лечения Большинство данных/мнений в пользу эффективности/пользы диагностической процедуры, вмешательства, лечения Эффективность/польза диагностической процедуры, вмешательства, лечения установлены менее убедительно |
Целесообразно применять Можно применять |
|
III |
Данные или единое мнение, что диагностическая процедура, вмешательство, лечение бесполезны/неэффективны, а в ряде случаев могут приносить вред |
Не рекомендуется применять |
Сокращение: ЕОК — Европейское общество кардиологов.
Таблица 2
Уровни достоверности доказательств согласно рекомендациям ЕОК
|
Уровень достоверности доказательств ЕОК |
Определение |
|
A |
Данные многочисленных рандомизированных клинических исследований или метаанализов |
|
B |
Данные получены по результатам одного рандомизированного клинического исследования или крупных нерандомизированных исследований |
|
C |
Согласованное мнение экспертов и/или результаты небольших исследований, ретроспективных исследований, регистров |
Сокращение: ЕОК — Европейское общество кардиологов.
Таблица 3
Шкала оценки уровней достоверности доказательств (УДД) для методов диагностики (диагностических вмешательств)
|
УДД |
Определение |
|
1 |
Систематические обзоры исследований с контролем референсным методом или систематический обзор рандомизированных клинических исследований с применением метаанализа |
|
2 |
Отдельные исследования с контролем референсным методом или отдельные рандомизированные клинические исследования и систематические обзоры исследований любого дизайна, за исключением рандомизированных клинических исследований, с применением метаанализа |
|
3 |
Исследования без последовательного контроля референсным методом или исследования с референсным методом, не являющимся независимым от исследуемого метода или нерандомизированные сравнительные исследования, в т. ч. когортные исследования |
|
4 |
Несравнительные исследования, описание клинического случая |
|
5 |
Имеется лишь обоснование механизма действия или мнение экспертов |
Сокращение: УДД — уровень достоверности доказательств.
Таблица 4
Шкала оценки уровней достоверности доказательств (УДД) для методов профилактики, лечения и реабилитации (профилактических, лечебных, реабилитационных вмешательств)
|
УДД |
Определение |
|
1 |
Систематический обзор РКИ с применением метаанализа |
|
2 |
Отдельные РКИ и систематические обзоры исследований любого дизайна, за исключением РКИ, с применением метаанализа |
|
3 |
Нерандомизированные сравнительные исследования, в т. ч. когортные исследования |
|
4 |
Несравнительные исследования, описание клинического случая или серии случаев, исследования "случай-контроль" |
|
5 |
Имеется лишь обоснование механизма действия вмешательства (доклинические исследования) или мнение экспертов |
Сокращения: РКИ — рандомизированное клиническое исследование, УДД — уровень достоверности доказательств.
Таблица 5
Шкала оценки уровней убедительности рекомендаций (УУР) для методов профилактики, диагностики, лечения и реабилитации (профилактических, диагностических, лечебных, реабилитационных вмешательств)
|
УУР |
Расшифровка |
|
A |
Сильная рекомендация (все рассматриваемые критерии эффективности (исходы) являются важными, все исследования имеют высокое или удовлетворительное методологическое качество, их выводы по интересующим исходам являются согласованными) |
|
B |
Условная рекомендация (не все рассматриваемые критерии эффективности (исходы) являются важными, не все исследования имеют высокое или удовлетворительное методологическое качество и/или их выводы по интересующим исходам не являются согласованными) |
|
C |
Слабая рекомендация (отсутствие доказательств надлежащего качества; все рассматриваемые критерии эффективности (исходы) являются неважными, все исследования имеют низкое методологическое качество и их выводы по интересующим исходам не являются согласованными) |
Сокращение: УУР — уровень убедительности рекомендаций.
Порядок обновления клинических рекомендаций
Механизм обновления клинических рекомендаций предусматривает их систематическую актуализацию — не реже чем 1 раз в 3 года, а также при появлении новых данных с позиции доказательной медицины по вопросам диагностики, лечения, профилактики и реабилитации конкретных заболеваний, наличии обоснованных дополнений/замечаний к ранее утверждённым клиническим рекомендациям, но не чаще 1 раза в 6 мес.
Решение об обновлении принимает Минздрав России на основе предложений, представленных медицинскими некоммерческими профессиональными организациями. Сформированные предложения должны учитывать результаты комплексной оценки лекарственных препаратов, медицинских изделий, а также результаты клинической апробации.
Приложение А3. Справочные материалы, включая соответствие показаний к применению и противопоказаний, способов применения и доз лекарственных препаратов, инструкции по применению лекарственного препарата
- Приказ Минздрава России от 15 ноября 2012г № 918н "Об утверждении Порядка оказания медицинской помощи больным с сердечно-сосудистыми заболеваниями" (с изменениями и дополнениями от 21.02.2020).
- Приказ Минздрава России от 02.03.2021 № 158н "Об утверждении стандарта медицинской помощи взрослым при остром коронарном синдроме без подъема сегмента ST электрокардиограммы (диагностика, лечение и диспансерное наблюдение)".
- Приказ Минздрава России от 15 марта 2022г № 168н "Об утверждении порядка проведения диспансерного наблюдения за взрослыми".
- "Методические рекомендации по проведению оценки научной обоснованности включаемой в клинические рекомендации информации" ФГБУ "ЦЭККМП" Минздрава России, 2019.
Критерии диагностики ИМ
Изложены согласно Четвертому универсальному определению ИМ [5].
Критерии диагностики острого ИМ
Термин "острый ИМ" используется в случаях, когда наряду с доказательством острого повреждения миокарда (характерная динамика уровня биомаркеров в крови) имеются свидетельства острой ишемии миокарда.
Критерии диагностики острого ИМ 1 и 2 типов
Повышение и/или снижение концентрации сердечного тропонина в крови, которая должна как минимум однократно превысить 99-й перцентиль верхней референсной границы у пациентов без исходного повышения уровня сердечного тропонина в крови, либо его увеличение >20% при исходно повышенном уровне сердечного тропонина, если до этого он оставался стабильным (вариация ≤20%) или снижался, в сочетании с хотя бы одним критерием острой ишемии миокарда.
Симптомы ишемии миокарда:
- Остро возникшие (или предположительно остро возникшие) ишемические изменения на ЭКГ;
- Появление патологических зубцов Q на ЭКГ;
- Подтверждение с помощью методов визуализации наличия новых участков миокарда с потерей жизнеспособности или нарушением локальной сократимости, характерных для ишемической этиологии;
- Выявление внутрикоронарного тромбоза при коронарной ангиографии или атеротромбоза (или признаков нестабильной АСБ) на аутопсии (для ИМ 1 типа).
Критерии дифференциальной диагностики ИМ 1 и 2 типов представлены на рис. 1.
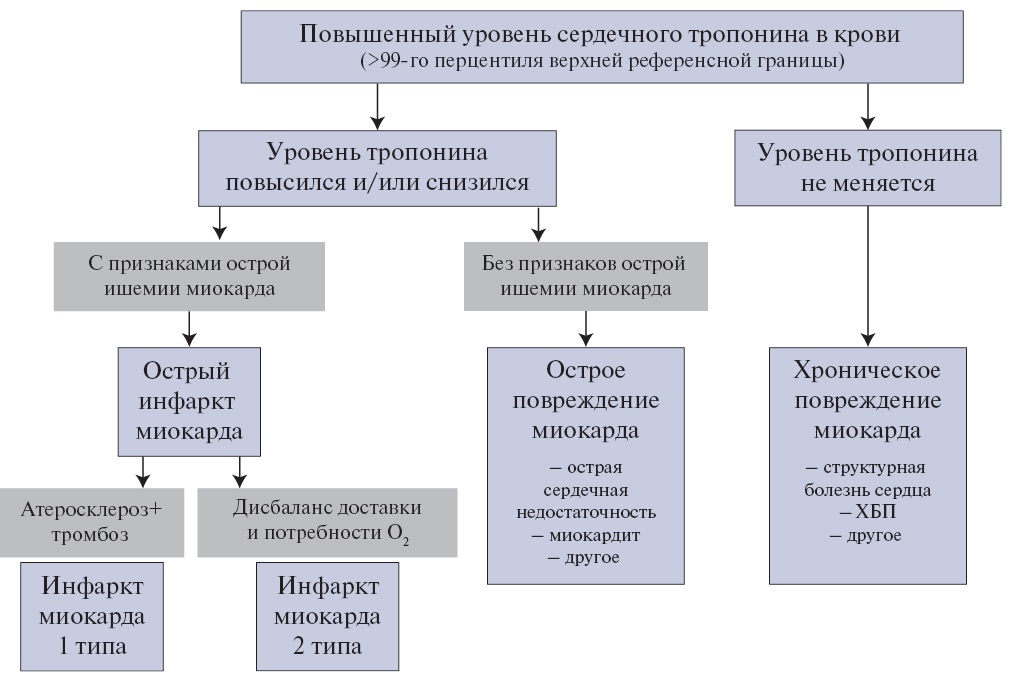
Рис. 1. Диагностика и дифференциальная диагностика острого ИМ 1 и 2 типов [5].
Сокращение: ХБП — хроническая болезнь почек.
Критерии диагностики острого ИМ 3 типа
Сердечная смерть у пациента с симптомами, указывающими на ишемию миокарда, сопровождающимися предположительно новыми ишемическими изменениями ЭКГ или ФЖ, в случаях, когда пациент умирает до появления возможности взятия образцов крови или раньше, чем отмечается повышение активности биохимических маркеров некроза миокарда в крови, или наличие ИМ подтверждено на аутопсии. При выявлении на аутопсии ИМ в сочетании со свежим или недавно возникшим атеротромбозом (или признаками нестабильной АСБ) в инфаркт-связанной КА ИМ 3 типа должен быть реклассифицирован в ИМ 1 типа.
Критерии диагностики острого ИМ 4а типа (первые 48 ч после процедуры ЧКВ)
Повышение концентрации сердечного тропонина в крови >5 раз от 99-го перцентиля верхней референсной границы у пациентов с исходно нормальным уровнем в крови (если до процедуры концентрация тропонина в крови была повышена и стабильна (вариация ≤20%) или снижалась, после процедуры он должен повыситься >20%) в сочетании как минимум с одним из признаков острой ишемии миокарда:
- Остро возникшие ишемические изменения ЭКГ;
- Появление патологических зубцов Q на ЭКГ;
- Подтверждение с помощью методов визуализации наличия новых участков миокарда с потерей жизнеспособности или нарушением локальной сократимости в виде паттерна, характерного для ишемической этиологии;
- Ангиографические признаки, указывающие на ограничения коронарного кровотока, связанные с процедурой (диссекция КА, окклюзия/тромбоз крупной эпикардиальной/боковой ветви, разрушение коллатерального кровотока или дистальная эмболизация);
- Посмертное выявление тромба, связанного с процедурой, в целевой артерии, или область некроза в миокарде, кровоснабжаемом этой артерией.
Критерии диагностики острого ИМ 4b типа
Критерии острого ИМ 1 типа в сочетании с тромбозом стента для КА, документированным при КГ или на аутопсии.
Критерии диагностики острого ИМ 4с типа
Критерии острого ИМ 1 типа, когда при КГ единственной причиной возникновения ИМ представляется рестеноз (не выявляются другие поражения, потенциально связанные с развившимся ИМ, нет признаков внутрикоронарного тромбоза).
Критерии диагностики острого ИМ 5 типа (первые 48 ч после операции КШ)
Повышение концентрации сердечного тропонина в крови >10 раз от 99-го перцентиля от верхней референсной границы у пациентов с исходно нормальным уровнем в крови (если до процедуры концентрация тропонина в крови была повышена и стабильна (вариация ≤20%) или снижалась, после процедуры он должен повыситься >20%) в сочетании как минимум с одним из признаков острой ишемии миокарда:
- Появление патологических зубцов Q на ЭКГ;
- Подтверждение с помощью методов визуализации наличия новых участков миокарда с потерей жизнеспособности или нарушением локальной сократимости в виде паттерна, характерного для ишемической этиологии;
- Острая окклюзия шунта или нативной КА, документированная при коронарной ангиографии.
Для биохимической диагностики острого ИМ должны использоваться методы определения концентрации сердечного тропонина в крови, обеспечивающие коэффициент вариации определений 99-го перцентиля верхней референсной границы не выше 20% (оптимально — не выше 10%).
Критерии диагностики ранее перенесенного ИМ
- Патологические зубцы Q на ЭКГ (с наличием предшествующих симптомов или без них) при отсутствии неишемических причин для их появления.
- Подтверждение с помощью методов визуализации наличия участков миокарда с потерей жизнеспособности, характерных для ишемической этиологии.
- Морфологические находки, характерные для перенесенного ИМ.
Причины повышения концентрации сердечного тропонина в крови
|
Повреждение (некроз) миокарда из-за острой ишемии миокарда — Изъязвление/разрыв атеросклеротической бляшки с тромбозом |
|
|
Повреждение (некроз) миокарда из-за острой ишемии миокарда за счет дисбаланса потребности и доставки кислорода |
|
|
Снижение перфузии миокарда: — Спазм КА — Дисфункция микрососудов — Эмболия в КА — Диссекция КА — Устойчивая брадиаритмия — Гипотония или шок — Дыхательная недостаточность — Тяжелая анемия |
Повышение потребности миокарда в кислороде: — Устойчивая тахиаритмия — Тяжелая гипертония с или без гипертрофии ЛЖ |
|
Другие причины повреждения (некроза) миокарда |
|
|
Сердечные причины: — Сердечная недостаточность — Миокардит — Кардиомиопатия (любая) — Синдром такоцубо — Процедуры реваскуляризации миокарда — Другие вмешательства на сердце — Катетерная аблация — Дефибрилляция — Контузия сердца |
Несердечные причины: — Сепсис, инфекционное заболевание — ХБП — Инсульт — Субарахноидальное кровоизлияние — ТЭЛА, легочная гипертензия — Инфильтративные заболевания — Химиотерапевтические препараты — Критические состояния — Тяжелая физическая нагрузка |
Примечание: изложены согласно Четвертому универсальному определению ИМ [5].
Сокращения: КА — коронарная артерия, ЛЖ — левый желудочек, ТЭЛА — тромбоэмболия легочной артерии, ХБП — хроническая болезнь почек.
Алгоритм исключения и подтверждения острого повреждения миокарда с учетом уровней сердечного тропонина в крови, определенных высокочувствительными методами при госпитализации и через 1 или 2 ч
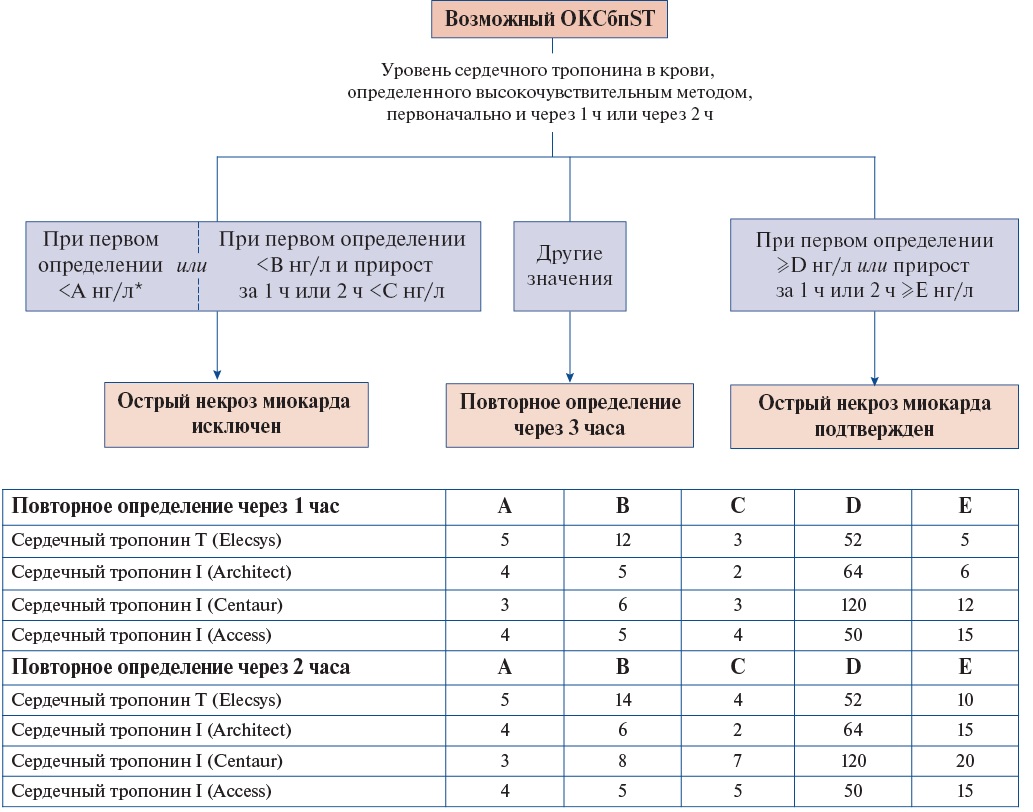
Примечание: * — если от начала боли >3 ч. Представлены лабораторные методы определения реагентами разных фирм, проверенные в рамках данного протокола [25-42]. Если после двух определений уровня сердечного тропонина в крови высокочувствительным методом с интервалом в 1 ч или 2 ч ни подтвердить, ни отвергнуть ИМ не удается, при сохраняющемся клиническом подозрении на ОКС рекомендуется дополнительное определение через 3 ч. Данный алгоритм позволяет выявить острое повреждение миокарда вне зависимости от его причины. Для диагностики острого ИМ необходимо наличие критериев, соответствующих Четвертому универсальному определению ИМ (Приложение А3. Критерии диагностики ИМ) [5].
Сокращение: ОКСбпST — острый коронарный синдром без стойкого подъема сегмента ST на ЭКГ.
Алгоритм исключения и подтверждения острого повреждения миокарда с учетом уровней сердечного тропонина в крови, определенных высокочувствительными методами при госпитализации и через 3 ч
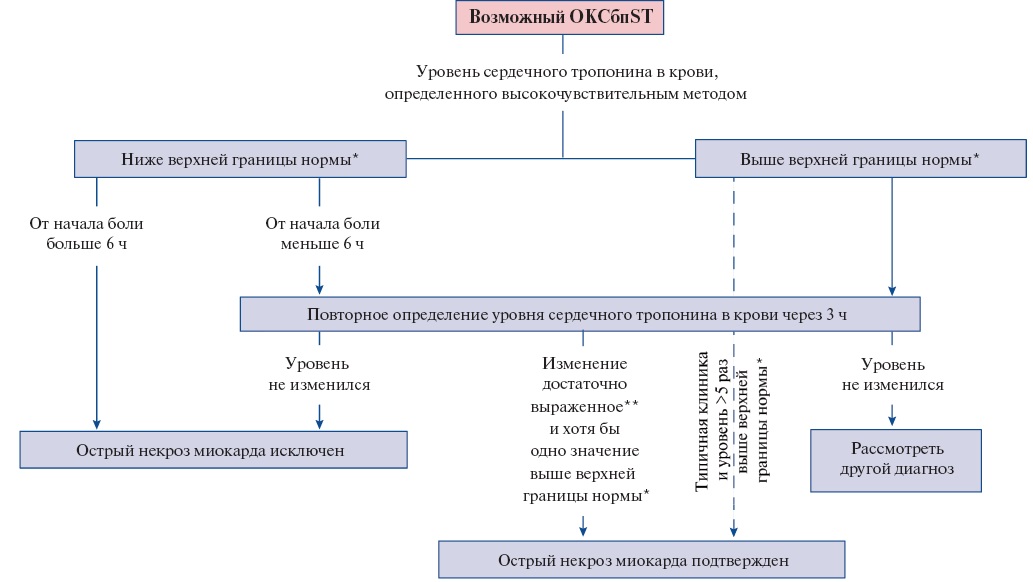
Примечание: * — 99-й перцентиль верхней референсной границы для данного метода определения, ** — величина изменения зависит от метода определения сердечного тропонина [25-42].
Сокращение: ОКСбпST — острый коронарный синдром без стойкого подъема сегмента ST на ЭКГ.
Изменения ЭКГ, влияющие на лечение пациента с ОКСбпST
|
Признак |
Изменение подхода к лечению пациента |
|
ФП, в т. ч. на ЭКГ, зафиксированных ранее |
Вероятная необходимость постоянного приема антикоагулянта. В дополнение к АСК** в большинстве случаев рекомендуется использовать клопидогрел** |
|
Тахикардия, особенно сохраняющаяся после купирования болевого синдрома |
Может быть как реакцией на боль, так и признаком сердечной недостаточности. В последнем случае раннее начало применения β-АБ (особенно его в/в введение) увеличивает риск развития кардиогенного шока. В этом случае до начала использования β-АБ следует оценить сократительную способность и ФВ ЛЖ, и если они снижены — отложить начало применение препаратов этой группы или первоначально использовать небольшие дозы |
|
Брадикардия |
Оценить наличие противопоказаний к использованию β-АБ, верапамила**, дилтиазема. Попытаться выяснить причину брадикардии. Оценить наличие показаний к установке временного ЭКС |
|
Нарушение атриовентрикулярной проводимости |
Оценить наличие противопоказаний к использованию β-АБ, верапамила**, дилтиазема. Оценить наличие показаний к установке временного ЭКС |
|
Желудочковые нарушения ритма |
При наличии жизнеугрожающих нарушений сердечного ритма или очевидной связи желудочковых аритмий с ишемией рекомендуется ранняя инвазивная стратегия ведения пациента |
|
Оценка динамики сегмента ST и зубцов T |
Требуется оценка всех имеющихся ЭКГ для выявления признаков, характерных для острой ишемии миокарда |
|
Блокада левой ножки пучка Гиса |
Усложняет отнесение пациента к категории ОКСпST и ОКСбпST. При отсутствии в анамнезе данных о наличии блокады левой ножки пучка Гиса рекомендуется констатировать наличие ОКСпST |
|
Удлинение интервала QT |
Может быть следствием электролитных расстройств, приема лекарственных средств, удлиняющих QT. Является ограничением для использования препаратов, удлиняющих QT. Необходимо нормализовать уровень калия и магния в крови, избегать выраженной брадикардии |
Сокращения: АСК — ацетилсалициловая кислота, ЛЖ — левый желудочек, ОКСбпST — острый коронарный синдром без стойкого подъема сегмента ST на ЭКГ, ОКСпST — острый коронарный синдром со стойким подъемом сегмента ST на ЭКГ, ФВ — фракция выброса, ФП — фибрилляция предсердий, ЭКГ — электрокардиография (син.: регистрация электрокардиограммы), электрокардиограмма, ЭКС — электрокардиостимулятор (электрокардиостимулятор имплантируемый двухкамерный, без частотной адаптации***; электрокардиостимулятор имплантируемый двухкамерный, частотно-адаптивный***; электрокардиостимулятор имплантируемый однокамерный, без частотной адаптации***; электрокардиостимулятор имплантируемый однокамерный, частотно-адаптивный***; электрокардиостимулятор имплантируемый трехкамерный (бивентрикулярный)***; электрокардиостимулятор имплантируемый двухкамерный, частотно-адаптивный, совместимый с магнитно-резонансным томографом***), β-АБ — бета-адреноблокаторы.
Изменения на ЭКГ у пациентов с ОКСбпST, указывающие на окклюзирующее поражение КА [3]
|
ЭКГ-синдром |
Изменения на ЭКГ в стандартных 12 отведениях |
Коронарная анатомия |
Локализация зоны ишемии/повреждения |
|
Окклюзия ствола ЛКА или многососудистое поражение в бассейне ЛКА |
1. Депрессия сегмента ST ≥1 мм в ≥6 отведениях (с вовлечением отведений нижнебоковой локализации) в сочетании с 2. Подъемом сегмента ST в aVR и/или V1. Сочетание данных ЭКГ-изменений с гемодинамической нестабильностью повышает вероятность окклюзирующего поражения ствола ЛКА или многососудистого поражения в бассейне ЛКА
|
Окклюзия ствола ЛКА или многососудистое поражение ветвей ЛКА |
Большая зона поражения ЛЖ |
|
Паттерн (феномен) де Винтера |
1. В отведениях V1-V6 косовосходящая депрессия (0,1-0,3 мВ (1-3 мм) в точке J) сегмента ST, которая продолжается в положительный высокий симметричный зубец T
|
Окклюзия/выраженный стеноз проксимального сегмента ПМЖА |
Передний сегмент стенки ЛЖ |
|
Синдром Велленса |
1. Точка J расположена на изолинии или минимально (<0,1 мВ (<1 мм)) приподнята в сочетании с 2. Двуфазным зубцом T в V2 и V3 (тип А) или 3. Симметричным глубоким отрицательным зубцом T в V2 и V3, иногда — в V1, V4, V5 или V6 (тип B)
|
Окклюзия/выраженный стеноз проксимального сегмента ПМЖА |
Передний сегмент стенки ЛЖ |
|
1-ая диагональная ветвь ПМЖА |
Сочетание признаков: 1. Подъем сегмента ST в aVL (иногда в I); 2. "Вертикальный" зубец T в aVL и V2; 3. Депрессия сегмента ST и инверсия зубца T в III и aVF. По взаимному расположению отведений ЭКГ с типичными изменениями данный ЭКГ-феномен известен как "признак южноафриканского флага"
|
Окклюзия/выраженный стеноз 1-ой диагональной ветви ПМЖА |
Передне-боковой (высокий боковой) сегмент стенки ЛЖ |
|
ИМ задней стенки ЛЖ |
1. Депрессия сегмента ST в V1-V3, особенно при положительном зубце T в этих же отведениях. Необходимо снять дополнительные отведения (V7-V9) для подтверждения/исключения прямых признаков поражения задней стенки ЛЖ (диагностически значимым в отведениях V7-V9 считается подъем сегмента ST ≥0,05 Мв (0,5 мм)).
|
Окклюзия/выраженный стеноз или дистального сегмента ОА, или ЗМЖА (ветви ПКА) |
Задний сегмент ЛЖ |
Сокращения: ЗМЖА — задняя межжелудочковая артерия, ИМ — инфаркт миокарда, ЛЖ — левый желудочек, ЛКА — левая коронарная артерия, ОА — огибающая артерия, ПКА — правая коронарная артерия, ПМЖА — передняя межжелудочковая артерия, ЭКГ — электрокардиография (син.: регистрация электрокардиограммы), электрокардиограмма.
Категории риска неблагоприятного исхода при ОКСбпST
|
Очень высокий риск |
|
— Нестабильность гемодинамики или кардиогенный шок |
|
— Продолжающаяся или повторяющаяся боль в грудной клетке, рефрактерная к медикаментозному лечению |
|
— Угрожающие жизни аритмии или остановка кровообращения |
|
— Острая сердечная недостаточность, предположительно связанная с сохраняющейся ишемией миокарда |
|
— Механические осложнения острого ИМ (разрыв свободной стенки ЛЖ, разрыв межжелудочковой перегородки, разрыв папиллярных мышц или хорд створок митрального клапана) |
|
— Повторяющиеся динамические смещения (особенно подъем) сегмента ST или изменения зубца T, предполагающие наличие ишемии миокарда |
|
Высокий риск |
|
— Наличие ИМбпST |
|
— Динамические смещения сегмента ST или изменения зубца T |
|
— Преходящий подъем сегмента ST |
|
— Сумма баллов по шкале GRACE 1.0 >140 |
Сокращения: ИМ — инфаркт миокарда, ИМбпST — инфаркт миокарда без подъема сегмента ST на ЭКГ, ЛЖ — левый желудочек.
Медикаментозное лечение ОКСбпST
|
Препарат |
Рекомендуемая доза |
|
Антиагреганты (АТХ-группа Антиагреганты, кроме гепарина, B01AC) |
|
|
АСК** [3] |
Внутрь; первая доза 150-300 мг (разжевать и проглотить), со 2-х суток 75-100 мг 1 раз/сут. |
|
Клопидогрел** |
Внутрь; первая доза 300 мг, со 2-х суток 75 мг 1 раз/сут. При планируемом ЧКВ: внутрь; нагрузочная доза 600 мг, затем 75 мг 1 раз/сут. |
|
Прасугрел |
Внутрь; 60 мг, со следующих суток по 10 мг 1 раз/сут. (у пациентов в возрасте ≥75 лет, с массой тела ниже 60 кг — по 5 мг 1 раз/сут.) |
|
Тикагрелор** |
Внутрь; первая доза 180 мг, через 12 ч по 90 мг 2 раза/сут.; при продлении лечения через 1 год после ИМбпST у пациентов с высоким риском коронарных осложнений — по 60 мг 2 раза/сут. |
|
F(ab')2 фрагменты антител моноклональных FRaMon |
В/в; 0,25 мг/кг в течение 3-5 мин |
|
Тирофибан |
В/в; начальная доза 25 мкг/кг в виде болюсной инъекции в течение 3 мин, с последующей инфузией со скоростью 0,15 мкг/кг/мин в течение 12-24 ч (минимальная длительность — 12 ч) и до 48 ч [3] |
|
Эптифибатид |
При ЧКВ: в/в болюсно 180 мкг/кг, затем в виде непрерывной инфузии по 2 мкг/кг/мин (при уровне креатинина сыворотки <1,912 ммоль/л) или 1 мкг/кг/мин (при уровне креатинина сыворотки 1,912-3,824 ммоль/л). Через 10 мин после начала инфузии повторно вводят 180 мкг/кг в виде болюса. Инфузию продолжают в течение 18-24 ч или до момента выписки больного из стационара, если она происходит раньше, но не менее 12 ч |
|
Антикоагулянты для парентерального введения |
|
|
Бивалирудин |
При начале введения до ЧКВ: в/в, болюсом 0,1 мг/кг с последующей инфузией 0,25 мг/кг/ч продолжительностью ≤72 ч; перед ЧКВ дополнительно болюсом 0,5 мг/кг с последующей инфузией 1,75 мг/кг/ч до окончания процедуры (при необходимости инфузию можно продолжить в той же дозе в течение 4 ч). При начале введения непосредственно перед ЧКВ: в/в болюсом 0,75 мг/кг с последующей инфузией 1,75 мг/кг/ч до окончания процедуры (при необходимости инфузию можно продолжить в той же дозе до 4 ч). Если до ЧКВ проводилась в/в инфузия НФГ**, ее следует прекратить и начать вводить бивалирудин через 30 мин |
|
Нефракционированный гепарин (гепарин натрия**) |
В/в, болюсом 60-70 ЕД/кг (максимально 5000 ЕД), сразу вслед за этим инфузия с начальной скоростью 12-15 ЕД/кг/ч (максимально 1000 ЕД/ч); в последующем подбор дозы, обеспечивающей увеличение АЧТВ в 1,5-2,5 раза выше контрольной величины для конкретной лаборатории (верхняя граница нормы или среднее нормальное значение у здоровых лиц). При ЧКВ: НФГ** (гепарина натрия**) вводится в/в болюсно в дозе 70-100 ЕД/кг (если не планируется применение ингибиторов ГП IIb/IIla рецепторов) или в дозе 50-60 ЕД/кг (при совместном применении с ингибиторами ГП IIb/IIIa рецепторов). При необходимости повторные болюсы с целью поддерживать активированное время свертывания крови 250-350 с (200-250 с при применении ингибиторов ГП IIb/IIIa тромбоцитов) |
|
Препарат |
Рекомендуемая доза |
|
Фондапаринукс натрия |
Подкожно 2,5 мг 1 раз/сут.; перед ЧКВ на фоне применения фондапаринукса ввести НФГ** в/в болюсом 85 ЕД/кг, при необходимости повторные болюсы с целью поддерживать активированное время свертывания крови 250-350 с (200-250 с при применении ингибиторов ГП IIb/IIIa тромбоцитов) |
|
Эноксапарин натрия** |
Подкожно 1 мг/кг 2 раза/сут. Особенности при почечной недостаточности: у пациентов со скоростью клубочковой фильтрации <30 мл/мин/1,73 м² подкожно 1 мг/кг 1 раз/сут. При ЧКВ на фоне подкожного введения эноксапарина натрия**: если до ЧКВ было сделано как минимум две подкожных инъекции эноксапарина натрия**, в первые 8 ч после очередной инъекции дополнительного антикоагулянта не требуется; если до ЧКВ была сделана только одна подкожная инъекция эноксапарина натрия** или ЧКВ выполняется через 8-12 ч после подкожной инъекции — ввести в/в болюсом 0,3 мг/кг эноксапарина натрия** [234][302][305][306]; если ЧКВ выполняется через >12 ч после подкожной инъекции — возможно применение любого антикоагулянта. #Перед ЧКВ у пациентов, до этого не получавших антикоагулянты (B01A Антитромботические средства): в/в болюсом 0,5 мг/кг, при процедуре длительностью >2 ч дополнительный болюс 0,25 мг/кг [607][608]. Данная доза применяется в т. ч. у пациентов, принимающих прямые оральные антикоагулянты (АТХ-группы Прямые ингибиторы тромбина, В01АЕ и Прямые ингибиторы фактора Ха, В01АF). Профилактика венозного тромбоза и ТЭЛА: под кожу живота, 40 мг 1 раз/сут. (если нет необходимости в более высоких дозах антикоагулянтов) |
|
Антикоагулянты для приема внутрь |
|
|
Антикоагулянты непрямые (антагонисты витамина К) |
Внутрь; доза подбирается индивидуально для поддержания МНО в границах целевого диапазона не менее 70%: — 2,0-2,5 в сочетании с антиагрегантами (АТХ-группа Антиагреганты, кроме гепарина, B01AC); — 2,5-3,5 в качестве монотерапии. Полное антитромботическое действие проявляется через 5 сут. после начала подбора дозы. Дозу можно считать условно подобранной, если два последовательных дня МНО находится в границах целевого диапазона (в последующем может потребоваться ее дальнейшая коррекция, чтобы добиться минимальных колебаний МНО в границах целевого диапазона) |
|
Апиксабан** |
Внутрь; 5 мг 2 раза/сут. (2,5 мг 2 раза/сут. при наличии как минимум 2 критериев — возраст ≥80 лет, масса тела ≤60 кг, уровень креатинина в крови ≥133 мкмоль/л) у пациентов с неклапанной ФП (в т. ч. после стентирования КА) |
|
Дабигатрана этексилат** |
Внутрь; 110 мг 2 раза/сут. или 150 мг 2 раза/сут. у пациентов с неклапанной ФП (в т. ч. после стентирования КА) |
|
Ривароксабан** |
Внутрь; доза зависит от показаний к применению и функции почек: — 2,5 мг 2 раза/сут. в сочетании с антиагрегантами (АТХ-группа Антиагреганты кроме гепарина, B01AC) у пациентов, не имеющих показаний к длительному применению антикоагулянтов для профилактики или лечения тромбоэмболических осложнений; — 15 мг 1 раз/сут. (#10 мг 1 раз/сут. при клиренсе креатинина 30-49 мл/мин) после стентирования КА у пациентов с неклапанной ФП (в сочетании с антиагрегантом (АТХ-группа Антиагреганты, кроме гепарина, B01AC)); — 20 мг 1 раз/сут. (15 мг 1 раз/сут. при клиренсе креатинина 30-49 мл/мин) у пациентов с неклапанной ФП |
|
β-АБ^,^^ |
|
|
#Карведилол^^^,** |
Внутрь; начальная доза 3,125-6,25 мг 2 раза/сут., при хорошей переносимости дозу увеличивают с интервалом 3-10 сут. до 25 мг 2 раза/сут. У пациентов с ХСН и другими ограничениями к применению β-АБ целесообразна медленная титрация дозы |
|
Метопролол** |
1. В/в медленно под контролем ЭКГ и АД по 5 мг каждые 2 мин до максимальной дозы 15 мг; при хорошей переносимости через 15 мин после последнего в/в введения прием внутрь до 50 мг каждые 6 ч в течение 48 ч. 2. Внутрь. Целевая доза метопролола при ИМ 100 мг в 2 раз/сут., при применении пролонгированных форм — 200 мг 1 раз/сут. Начальная доза 12,5-25 мг 2 раза/сут. у пациентов с ХСН и другими ограничениями к применению β-АБ |
|
Эсмолол |
При наджелудочковой тахикардии (за исключением синдрома предвозбуждения желудочков), когда другие в/в или пероральные β-АБ нецелесообразно или невозможно применить, в/в инфузия под контролем ЭКГ и АД; нагрузочная доза 0,5 мг/кг в течение 1 мин, затем 0,05 мг/кг/мин в течение 4 мин, при недостаточном эффекте увеличение скорости инфузии на 0,05 мг/кг/мин каждые 4 мин вплоть до 0,2 мг/кг/мин; если необходим более быстрый эффект, перед 2-м и 3-м увеличением дозы ввести дополнительные болюсы по 0,5 мг/кг. Гемодинамический эффект сохраняется 20-30 мин после прекращения введения. При переходе на прием других β-АБ внутрь через 1 ч после их первого назначения необходимо снизить дозу эсмолола на 50%; обычно эсмолол отменяют после перорального приема второй дозы β-АБ, если за это время поддерживались надлежащие ЧСС и АД |
|
Ингибиторы ангиотензинпревращающего фермента: лечение с 1-х суток заболевания§ |
|
|
Каптоприл** |
Внутрь; первая доза 6,25 мг, через 2 ч 12,5 мг, через 12 ч 25 мг. Целевая доза 100 мг в сутки за 2 приема на 4 нед. По истечении 4 нед. следует вновь оценить состояние пациента и при хорошей переносимости продолжить терапию в дозе 50 мг 3 раза/сут. |
|
Лизиноприл** |
Внутрь; первая доза 5 мг, через 24 ч — 5 мг; целевая доза 10 мг 1 раз/сут. |
|
Зофеноприл |
Внутрь; 7,5 мг 2 раза/сут. в 1-2-е сутки, при хорошей переносимости 15 мг 2 раза/сут. на 3-4-е сутки, затем 30 мг 2 раза/сут. |
|
Ингибиторы ангиотензинпревращающего фермента: лечение с более отдаленных сроков заболевания§ |
|
|
Каптоприл&,** |
Внутрь; целевая доза 100 мг в сутки за 2 приема на 4 нед. По истечении 4 нед. следует вновь оценить состояние пациента и при хорошей переносимости продолжить терапию в дозе 50 мг 3 раза/сут. |
|
Препарат |
Рекомендуемая доза |
|
Рамиприл**,&,&& |
Внутрь; начальная доза: 2,5 мг 1 раз/сут. Рекомендуется удваивать дозу с интервалом 1-2 нед.; целевая доза 5 мг 2 раза/сут. |
|
Эналаприл&,** |
Внутрь; начальная доза 2,5 мг; целевая доза 10 мг 2 раза/сут. |
|
Антагонисты рецепторов ангиотензина II# |
|
|
Валсартан |
Внутрь; начальная доза 20 мг 2 раза/сут. с постепенным увеличением до 160 мг 2 раза/сут. |
|
Антагонисты минералокортикоидных рецепторов (АТХ-группа Антагонисты альдостерона, C03DA)§ |
|
|
Эплеренон&&& |
Внутрь; первая доза 25 мг 1 раз/сут., при хорошей переносимости у пациентов, не принимающих амиодарон**, дилтиазем или верапамил**, в ближайшие 4 нед. увеличение дозы до 50 мг 1 раз/сут. |
|
Органические нитраты |
|
|
Нитроглицерин** |
В/в инфузия 5-166 мкг/мин; обычно сначала инфузия 10-20 мкг/мин с возможным увеличением на 5-10 мкг/мин каждые 5-10 мин до уменьшения симптомов и/или снижения систолического АД на 10-15% при исходно нормальном АД и на 25-30% при АГ (но не ниже 95 мм рт.ст.) |
Примечания: в таблице представлены лекарственные средства, наиболее изученные при ОКС. Не исключено применение других лекарственных средств с аналогичным механизмом действия.
В пределах каждой группы препараты перечислены по алфавиту.
^ — могут использоваться и другие препараты в надлежащих дозах, не обладающие внутренней симпатомиметической активностью; ^^ — в каждом конкретном случае дозы β-АБ могут быть меньше или несколько выше в зависимости от индивидуальной переносимости и клинического эффекта у конкретного пациента; у пациентов с ХСН при существенно нарушенной сократительной функции ЛЖ положительное влияние на выживаемость показано для бисопролола в целевой дозе 10 мг 1 раз/сут., карведилола** в целевой дозе 25 мг 2 раза/сут. и метопролола** пролонгированного действия в целевой дозе 200 мг 1 раз/сут.; ^^^ — у пациентов с ИМ и существенно нарушенной сократительной функцией ЛЖ (ФВ ≤40%) показано положительное влияние на смертность; § — указаны препараты с положительным влиянием на смертность после ИМ; особенности подбора дозы у конкретного пациента зависят от реакции АД, уровня креатинина и калия в крови; если достичь целевой дозы препарата не удается, следует использовать максимально переносимую дозу; & — доказательства положительного влияния на прогноз получены у пациентов с СН (в т. ч. преходящей в ранние сроки ИМ) и/или ФВ ЛЖ <40%; && — доказательства положительного влияния на прогноз получены у пациентов без выраженного снижения сократительной способности ЛЖ; &&& — при недоступности эплеренона можно использовать #спиронолактон** в тех же дозах [124][609].
Сокращения: АГ — артериальная гипертензия, АД — артериальное давление, АСК — ацетилсалициловая кислота, АЧТВ — активированное частичное тромбопластиновое время, в/в — внутривенно, ГП IIb/IIIa — гликопротеины IIb/IIIa, ЕД (ед.) — единицы действия, ИМбпST — инфаркт миокарда без подъема сегмента ST на ЭКГ, КА — коронарная артерия, ЛЖ — левый желудочек, МНО — международное нормализованное отношение, НФГ — нефракционированный гепарин (син.: гепарин натрия**), ОКС — острый коронарный синдром, ТЭЛА — тромбоэмболия легочных артерий, ФВ — фракция выброса, ФП — фибрилляция предсердий, ХСН — хроническая сердечная недостаточность, ЧКВ — чрескожное коронарное вмешательство (транслюминальная баллонная ангиопластика коронарных артерий; стентирование коронарной артерии; транслюминальная баллонная ангиопластика и стентирование коронарных артерий; реканализация коронарных артерий ретроградная со стентированием; реканализация коронарных артерий антеградная со стентированием; тромбоэктомия из сосудистого протеза (коронарного); эмболэктомия (из коронарной артерии); попытка стентирования коронарных артерий), ЧСС — частота сердечных сокращений, ЭКГ — электрокардиография (син.: регистрация электрокардиограммы), электрокардиограмма, β-АБ — бета-адреноблокаторы.
Дозы антитромботических лекарственных средств при нарушенной функции почек
|
Препарат |
рСКФ 30-59 мл/мин/1,73 м² |
рСКФ 15-29 мл/мин/1,73 м² |
рСКФ <15 мл/мин/1,73 м² |
|
|
Антиагреганты (АТХ-группа Антиагреганты, кроме гепарина, B01AC) |
||||
|
АСК** |
Неотложная помощь |
Доза не меняется |
||
|
Плановое назначение |
Согласно инструкции, АСК** противопоказана при тяжелом нарушении функции почек без уточнения уровня КлКр/рСКФ. В таких случаях рекомендуется индивидуальная оценка пользы/рисков назначения |
|||
|
Клопидогрел**¹ |
Неотложная помощь |
Обычная доза |
Обычная доза |
Нет данных. В таких случаях рекомендуется индивидуальная оценка пользы/рисков назначения с учетом данных инструкции² |
|
Плановое назначение |
Обычная доза |
Обычная доза |
||
|
Прасугрел |
Обычная доза |
Обычная доза |
Опыт применения прасугрела у пациентов с почечной недостаточностью ограничен. Для пациентов с почечной недостаточностью, включая пациентов с терминальной почечной недостаточностью, коррекции дозы не требуется. Рекомендации ЕОК использовать не рекомендуют |
|
|
Тикагрелор**³ |
Обычная доза |
Обычная доза |
Отсутствует информация о применении препарата у пациентов на гемодиализе, поэтому его применение у этих пациентов не показано. Не требуется корректировать дозу препарата у пациентов с почечной недостаточностью. Тем не менее Рекомендации ЕОК использовать не рекомендуют |
|
|
F(ab`)2 фрагменты антител моноклональных FRaMon |
В инструкции к препарату нет ограничений со стороны функции почек. Рекомендуется учитывать общий риск геморрагических осложнений |
|||
|
Тирофибан |
В инструкции к препарату нет ограничений со стороны функции почек. Рекомендуется учитывать общий риск геморрагических осложнений |
|||
|
Эптифибатид¹ |
КлКр ≥50 мл/мин: обычная доза. КлКр ≥30, но <50 мл/мин: доза для инфузии снижена до 1,0 мкг/кг/мин |
Данных нет. Клинические рекомендации ЕОК использовать не рекомендуют |
||
|
Антикоагулянты |
||||
|
Нефракционированный гепарин (гепарин натрия)** |
Доза подбирается под контролем АЧТВ независимо от фильтрационной функции почек |
|||
|
Эноксапарин натрия** |
Обычная доза |
Согласно инструкции, при тяжелой почечной недостаточности (без указания количественного уровня): у пациентов <75 лет: увеличить интервал между введением препаратов с 12 до 24 ч; у пациентов ≥75 лет и старше: увеличить интервал между введением препаратов с 12 до 24 ч, при этом доза на каждое введение — 1 мг/кг [2] |
Не рекомендуется (нет данных) |
|
|
Фондапаринукс натрия¹ |
Не рекомендован у пациентов с КлКр <20 мл/мин. Коррекция дозы не требуется у пациентов с КлКр ≥20 мл/мин |
|||
|
Бивалирудин |
Доза не меняется, за исключением пациентов, которым предстоит ЧКВ: скорость инфузии должна быть снижена до 1,4 мг/кг/ч (первоначальная доза 0,75 мг/кг, которую вводят струйно, не изменяется). Во время проведения ЧКВ рекомендуется проводить контроль времени свертывания, например АВС. Значение АВС необходимо проверять через 5 мин после струйного введения первоначальной дозы. Если величина АВС составляет <225 с, то необходимо повторно струйно ввести препарат в дозе 0,3 мг/кг и еще раз проверить АВС через 5 мин после введения повторной дозы |
Противопоказан |
||
|
Ривароксабан**¹ в дозе 2,5 мг 2 раза/сут. |
Доза без изменений, но применять с осторожностью при одновременном назначении препаратов, повышающих концентрацию ривароксабана в плазме крови |
Доза без изменений, но применять с осторожностью |
Противопоказан (данных нет) |
|
Примечание: ¹ — для данных препаратов, согласно инструкции, оценка функции почек с целью безопасности их назначения, должна производиться с использованием КлКр; ² — после повторных приемов клопидогрела** в дозировке 75 мг/сут. у пациентов с тяжелым поражением почек (КлКр — от 5 до 15 мл/мин) ингибирование аденозинфосфат-индуцированной агрегации тромбоцитов (25%) было ниже по сравнению с таковым у здоровых добровольцев, однако удлинение времени кровотечения сравнимо с данными у здоровых добровольцев, получавших клопидогрел** в дозировке 75 мг/сут.; ³ — в качестве превентивной меры следует избегать применения тикагрелора** у пациентов с гиперурикемической нефропатией.
Сокращения: АВС — активированное время свёртывания крови, АСК — ацетилсалициловая кислота**, АЧТВ — активированное частичное тромбопластиновое время, ЕОК — Европейское общество кардиологов, КлКр — клиренс креатинина, рСКФ — расчётная скорость клубочковой фильтрации, ЧКВ — чрескожное коронарное вмешательство (транслюминальная баллонная ангиопластика коронарных артерий; стентирование коронарной артерии; транслюминальная баллонная ангиопластика и стентирование коронарных артерий; реканализация коронарных артерий ретроградная со стентированием; реканализация коронарных артерий антеградная со стентированием; тромбоэктомия из сосудистого протеза (коронарного); эмболэктомия (из коронарной артерии); попытка стентирования коронарных артерий).
В/в инсулинотерапия при ОКСбпST
Показания для инсулинотерапии у пациентов ОКСбпST и СД
- СД 1 типа.
- Уровень глюкоза плазмы при поступлении и последующих определениях стойко >10 ммоль/л.
- Диабетический кетоацидоз.
- Гиперосмолярное гипергликемическое состояние.
- Известное лечение высокими дозами глюкокортикоидов.
- Парентеральное питание.
- Тяжелое/критическое состояние.
- Кардиогенный шок, выраженная застойная СН, тяжелая постинфарктная стенокардия, артериальная гипотензия, тяжелые нарушения сердечного ритма.
- Любая степень нарушения сознания.
Алгоритм для непрерывной в/в инфузии инсулинов
- Непрерывная в/в инфузия инсулинов проводится через отдельный инфузомат с применением раствора инсулинов и аналогов быстрого действия с концентрацией 1 ед./1 мл 0,9% раствора натрия хлорида**. В отсутствие инфузомата допускается в/в капельное введение.
- Рекомендуется определять уровень глюкозы в плазме крови 1 раз в час до ее стабилизации в выбранном целевом диапазоне минимум 4 ч; затем 1 раз в 2 ч в течение 4 ч; в дальнейшем — 1 раз в 4 ч. У пациентов в критическом состоянии требуется определять глюкозу в плазме крови 1 раз в час даже при стабильном целевом уровне.
- Рекомендуемая средняя начальная скорость непрерывной в/в инфузии инсулинов у пациентов, уже имеющих уровень глюкозы в плазме крови в целевом диапазоне — 0,5-1 ед./ч, для не находящихся в целевом диапазоне — 2-3 ед./ч (при наличии кетоацидоза — 0,1 ед./кг массы тела в час (но не >15 ед./ч). Более низкая начальная скорость (<0,5 ед./ч) используется при дефиците массы тела, почечной, печеночной или надпочечниковой недостаточности. Более высокая начальная скорость (>3 ед./ч) используется при сверхвысокой гипергликемии (>25 ммоль/л) и предполагаемой инсулинорезистентности (выраженном ожирении, признаках инфекции, хронической терапии глюкокортикоидами).
- Одновременно с непрерывной в/в инфузией инсулина желательно наладить инфузию 5-10% раствора декстрозы** (требуется вводить 5 г декстрозы** в час). Важно вводить инсулины и декстрозу** через разные инфузионные системы, т. к. требуется частая раздельная коррекция скорости инфузии каждого из двух растворов. При уровне глюкозы в плазме крови выше 14 ммоль/л декстроз** у не вводят (до следующего определения ее уровня).
- При уровне глюкозы в плазме крови <3,3 ммоль/л требуется остановить инфузию инсулинов и ввести в/в 30-60 мл 40% раствора декстрозы**, при необходимости повторять введение декстрозы** каждые 20 мин. После двукратного подтверждения уровня глюкозы плазмы >3,9 ммоль/л следует возобновить инфузию инсулинов с меньшей скоростью.
- При переходе на подкожное введение инсулинов их инфузию прекращают через 1-2 ч после первой подкожной инъекции инсулинов и аналогов быстрого действия, или через 2-3 ч после первой инъекции инсулинов и аналогов длительного действия.
Рекомендуемая скорость инфузии инсулина в зависимости от уровня глюкозы в плазме крови
|
Глюкоза (ммоль/л) |
<3,9 |
3,9-6,1 |
6,2-6,6 |
6,7-8,3 |
8,4-9,9 |
10-13,3 |
13,4-16,6 |
16,7-19,9 |
>20 |
|
Скорость введения инсулинов (ед./ч) |
Не вводить |
0,2 |
0,5 |
1 |
1,5 |
2 |
3 |
4 |
6 |
У отдельных пациентов (ранее получавших >80 ЕД инсулинов в сутки, получающих глюкокортикостероиды, не достигающих целевой гликемии при использовании предлагаемого алгоритма) может потребоваться использование более интенсивных алгоритмов. У пациентов с повторным определением уровня глюкозы в плазме крови <3,9 ммоль/л (два раза подряд) требуется перейти на менее интенсивный алгоритм. Ознакомиться с этими алгоритмами можно в рекомендациях по ведению пациентов с СД.
Классификация ОСН при ИМ по Killip [439][440]
|
Класс |
Признаки |
Госпитальная смертность |
|
I |
Нет признаков сердечной недостаточности |
2-3% |
|
II |
III тон, влажные хрипы в нижних отделах легких |
5-12% |
|
III |
Отек легких, влажные хрипы выше углов лопаток |
10-20% |
|
IV |
Кардиогенный шок |
50-81% |
Гемодинамические варианты кардиогенного шока
|
Волемический статус |
||
|
Мокрый |
Сухой |
|
|
Холодный |
Классический кардиогенный шок (↓СИ, ↑ОПСС, ↑ДЗЛК) |
Эуволемический кардиогенный шок (↓СИ, ↑ОПСС, нормальное ДЗЛК) |
|
Теплый |
Вазодилататорный кардиогенный шок или смешанный шок (↓СИ, ↓ОПСС, ↑ДЗЛК) |
Вазодилататорный (некардиогенный) шок (↑СИ, ↓ОПСС, ↓ДЗЛК) |
Примечание: ↓ — снижение, ↑ — повышение.
Сокращения: ДЗЛК — давление заклинивания легочных капилляров, ОПСС — общее периферическое сосудистое сопротивление, СИ — сердечный индекс.
Параметры для мониторирования у пациента с кардиогенным шоком
|
Параметр |
Номенклатура медицинских услуг |
Частота измерения |
Обоснование |
|
Кардиомониторинг, пульсоксиметрия, частота дыхательных движений |
А12.30.004 Суточное прикроватное мониторирование жизненноважных функций и параметров, А05.10.007 Мониторирование электрокардиографических данных, А05.12.008 Чрескожный мониторинг парциального давления кислорода, А02.12.001.001 Исследование пульса методом мониторирования, В01.003.003 Суточное наблюдение врачом-анестезиологом-реаниматологом, В03.003.005 Суточное наблюдение реанимационного пациента, В03.015.002 Контроль степени повреждения миокарда при инфаркте миокарда |
Непрерывно |
Высокий процент нарушений ритма, высокий риск отека легких, ИВЛ |
|
Инвазивное измерение АД (артериальная линия) |
А11.12.012 Катетеризация артерий конечностей, А02.12.002.002 Дистанционное наблюдение за показателями артериального давления, А14.12.001 Уход за сосудистым катетером |
Непрерывно |
Прекращение инвазивного мониторирования АД не ранее 12-24 ч после прекращения введения вазоактивных препаратов |
|
ЦВД |
А02.12.003 Измерение ЦВД, А11.12.001 Катетеризация подключичных и других центральных вен, А14.12.001 Уход за сосудистым катетером |
Не менее 4 раз в сутки |
Продленное мониторирование ЦВД позволяет оценивать волемический статус пациента |
|
Смешанная венозная сатурация |
А11.12.001 Катетеризация подключичных и других центральных вен, В03.016.011 Исследование кислотно-основного состояния и газов крови, А14.12.001 Уход за сосудистым катетером, В01.003.003 Суточное наблюдение врачом-анестезиологом-реаниматологом |
Каждые 4 ч |
Мониторирование смешанной венозной сатурации позволяет судить о изменении сердечного выброса и оценивать эффективность вазоактивной терапии |
|
Темп диуреза |
А11.28.007 Катетеризация мочевого пузыря, В03.003.005 Суточное наблюдение реанимационного пациента, А14.28.002 Уход за мочевым катетером |
Каждый час |
Темп диуреза и уровень креатинина сыворотки — показатель почечной перфузии |
|
Инвазивный мониторинг гемодинамики и неинвазивный мониторинг сердечного выброса |
А11.12.001 Катетеризация подключичных и других центральных вен, А11.10.005 Зондирование камер сердца, А24.12.001 Исследование гемодинамики методом термодилюции, А04.10.002 Эхокардиография, А12.10.003 Исследование сердечного выброса, А14.12.001 Уход за сосудистым катетером |
При необходимости |
Является обязательным при отсутствии эффективности стартовой терапии и неясности диагноза |
|
Общий (клинический) анализ крови |
В03.016.002 Общий (клинический) анализ крови, В03.016.003 Общий (клинический) анализ крови развернутый |
Каждые 12-24 ч |
Чаще для пациентов с возможным кровотечением |
|
Электролиты |
А09.05.031 Исследование уровня калия в крови, А09.05.127 Исследование уровня общего магния в сыворотке крови, А09.05.034 Исследование уровня хлоридов в крови, А09.05.030 Исследование уровня натрия в крови, А09.05.032 Исследование уровня общего кальция в крови |
Каждые 6-12 ч |
|
|
Креатинин |
А09.05.020 Исследование уровня креатинина в крови |
Каждые 12-24 ч |
|
|
АСТ, АЛТ, ЛДГ, билирубин |
А09.05.041 Определение активности аспартатаминотрансферазы в крови, А09.05.042 Определение активности аланинаминотрансферазы в крови, А09.05.039 Определение активности лактатдегидрогеназы в крови, А09.05.021 Исследование уровня общего билирубина в крови |
Ежедневно |
Мониторинг застоя в печени, острого печеночного повреждения |
|
Параметр |
Номенклатура медицинских услуг |
Частота измерения |
Обоснование |
|
Лактат |
В03.016.011 Исследование кислотно-основного состояния и газов крови |
Каждые 1-4 ч |
Клиренс лактата отражает тяжесть полиорганной недостаточности. Снижение выведения лактата ассоциировано с увеличением летальности |
|
Коагулограмма (ориентировочное исследование системы гемостаза) |
В03.005.006 Коагулограмма (ориентировочное исследование системы гемостаза) |
Каждые 4-6 ч для пациентов с продленной инфузией НФГ**, каждые 24 ч для пациентов без антикоагулянтов |
Сокращения: АД — артериальное давление, АЛТ — aланинаминотрансфераза, АСТ — аспартатаминотрансфераза, ИВЛ — искусственная вентиляция лёгких, ЛДГ — лактатдегидрогеназа, НФГ — нефракционированный гепарин натрия**, ЦВД — центральное венозное давление.
Стадии кардиогенного шока в зависимости от клинической тяжести и реакции на лечение
|
Стадии кардиогенного шока |
Клиническая характеристика |
|
Прешок |
У пациента нет признаков или симптомов кардиогенного шока, но есть риск развития — острый ИМ, признаки ОСН или декомпенсации ХСН |
|
Развернутая картина кардиогенного шока |
Признаки гипоперфузии тканей. Влажные хрипы в легких. Диурез <30 мл/ч. Уровень лактата от 2 ммоль/л и выше. При инвазивном мониторинге гемодинамики СИ <2,2 л/мин/м², ДЗЛК >15 мм рт.ст. |
|
Резистентный кардиогенный шок |
Состояние пациентов, симптомы и признаки шока как на предыдущей стадии, но не улучшаются на фоне лечения или ухудшаются. Уровень лактата сохраняется >2 ммоль/л и повышается. Показатели функции печени и почек ухудшаются |
Сокращения: ДЗЛК — давление заклинивания легочных капилляров, ИМ — инфаркт миокарда, ОСН — острая сердечная недостаточность, СИ — сердечный индекс, ХСН — хроническая сердечная недостаточность.
Стадии кардиогенного шока согласно классификации Американского общества сердечно-сосудистой ангиографии и интервенции (SCAI)
|
Стадии кардиогенного шока |
Характеристика пациентов |
|
E (extremis) |
Пациенты в состоянии остановки кровообращения и продолжающейся сердечно-легочной реанимации, которым проводится активная коррекция всеми методами, включая ЭКМО |
|
D (deteriorating) |
Пациенты с признаками кардиогенного шока, не отвечающими на терапию в течение 30 мин |
|
C (classic) |
Пациенты с гипоперфузией, требующей оценки и коррекции волемического статуса и лечения (инотропная, вазопрессорная или механическая поддержка кровообращения, ЭКМО) |
|
B (beginning) |
Пациенты с ИМ с гипотензией без признаков гипоперфузии |
|
A (at risk) |
Пациенты с острым ИМ |
Примечание: адаптировано из [610].
Сокращения: ИМ — инфаркт миокарда, ЭКМО — экстракорпоральная мембранная оксигенация.
Основные противопоказания к проведению физических тренировок
— ОКС;
— острая и подострая аневризма ЛЖ, подтвержденная инструментальными методами диагностики;
— наличие внутрисердечных тромбов;
— СН IV ФК по NYHA;
— нарушения сердечного ритма (желудочковые экстрасистолы и тахикардия опасных градаций, пароксизмальные тахиаритмии, возникающие при физической нагрузке), не корригируемые оптимальной терапией;
— нарушения проводимости (синоатриальная и АВ-блокады 2-3 степени, кроме пациентов с имплантированными ЭКС);
— неконтролируемая АГ (>180/110 мм рт.ст.; снижение систолического АД ≥20 мм рт.ст. при физической нагрузке);
— выраженный аортальный стеноз;
— тяжелая обструктивная гипертрофическая кардиомиопатия;
— острый перикардит, миокардит, эндокардит;
— диссекция аорты;
— синкопальные состояния;
— тромбоэмболия или тромбофлебит (в сроки до 3 мес.);
— острое нарушение мозгового кровообращения или транзиторная ишемическая атака (в сроки до 3 мес.);
— неконтролируемый СД;
— острое инфекционное заболевание (в т. ч. и вирусные инфекции);
— заболевания опорно-двигательного аппарата;
— тяжелая сопутствующая патология.
Приложение Б. Алгоритмы действий врача
Приложение Б1. Выбор стратегии ведения пациента с ОКСбпST
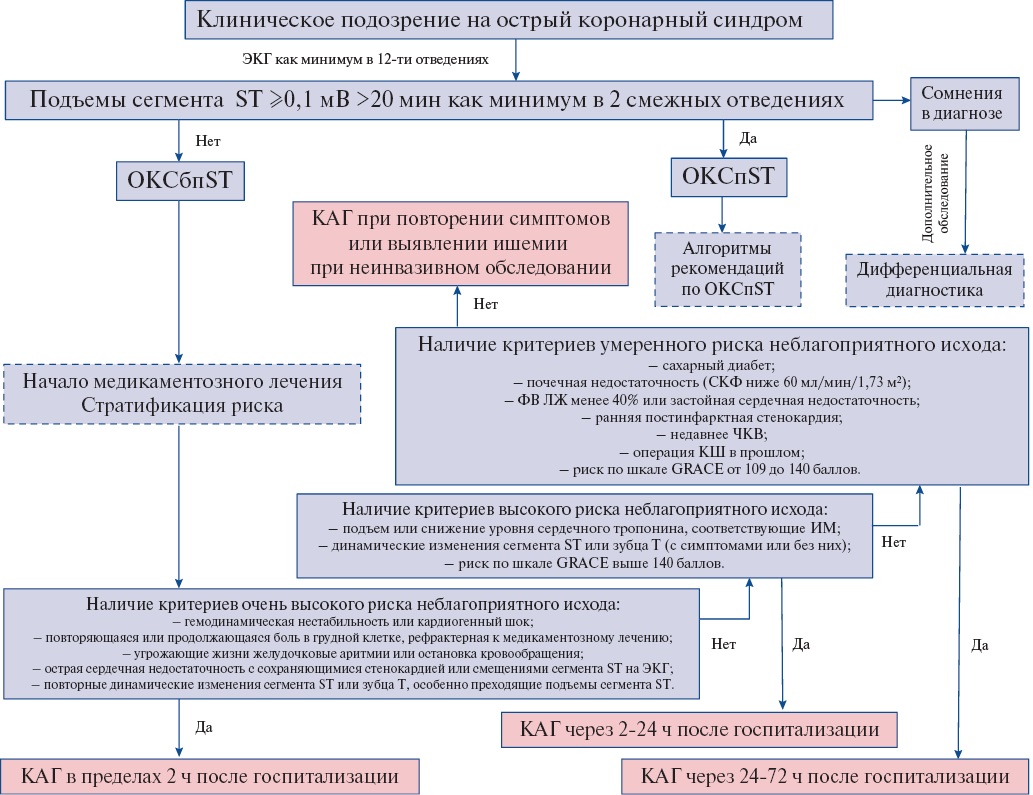
Сокращения: ИМбпST — инфаркт миокарда без подъема сегмента ST на ЭКГ, ОКС — острый коронарный синдром, ОКСбпST — острый коронарный синдром без стойкого подъема сегмента ST на ЭКГ, ОКСпST — острый коронарный синдром со стойким подъемом сегмента ST на ЭКГ, ЭКГ — электрокардиография (син.: регистрация электрокардиограммы), электрокардиограмма.
Приложение Б2. Догоспитальный алгоритм действий врача скорой медицинской помощи или фельдшера при ОКСбпST
- Рекомендуется сбор жалоб, анамнеза, оценка болевого синдрома, физикальное обследование, контроль показателей гемодинамики (АД, ЧСС).
- Рекомендуется ограничение двигательной активности.
- Рекомендуется регистрация ЭКГ как минимум в 12-ти отведениях и осуществление дистанционного консультирования ЭКГ (при необходимости и возможности проведения).
- Рекомендуется начать непрерывное мониторирование ЭКГ, наладить в/в доступ, обеспечить готовность к проведению дефибрилляции и сердечно-легочной реанимации.
- Рекомендуется экстренная госпитализация в стационар, включенный в систему маршрутизации больных с ОКС.
При наличии критериев высокого риска развития неблагоприятных ишемических событий (стойкий или рецидивирующий болевой синдром, смещения сегмента ST на ЭКГ, нестабильные гемодинамические параметры/шок, отек легких, угрожающие жизни ЖА/нарушения внутрисердечной проводимости или остановка кровообращения, подозрение на механические осложнения ИМ) показана экстренная госпитализация пациентов в стационар, где есть возможность выполнения ЧКВ в течение 2-х ч после госпитализации. Следует информировать принимающий стационар о транспортировке нестабильного пациента.
- Пациентам с ОКСбпST проведение тромболитической терапии не рекомендуется.
- Рекомендуется купирование болевого синдрома.
При отсутствии артериальной гипотонии и других противопоказаний рекомендуется нитроглицерин** в дозе 0,4-0,5 мг в виде таблеток под язык или аэрозоля (спрея). Если симптомы не исчезают через 5 мин, а препарат удовлетворительно переносится, можно использовать его повторно. В связи с опасностью артериальной гипотонии необходимо постоянно контролировать АД.
Если болевой синдром сохраняется после 3 приемов нитроглицерина**, необходимо в/в введение наркотического анальгетика. Доза наркотического анальгетика, необходимая для адекватного обезболивания, должна подбираться индивидуально. Препаратом выбора является морфин**. Перед использованием 10 мг морфина** разводят как минимум в 10 мл 0,9% раствора натрия хлорида**. Первоначально следует ввести в/в медленно 2-4 мг лекарственного вещества. При необходимости введение повторяют каждые 5-15 мин по 2-4 мг до купирования боли или возникновения побочных эффектов, не позволяющих увеличить дозу.
- При отсутствии противопоказаний рекомендуется рассмотреть применение ацетилсалициловой кислоты в дозе 150-300 мг (разжевать; не рекомендуется принимать внутрь кишечнорастворимые лекарственные формы препарата).
- Применение ингибитора P2Y12-рецептора тромбоцитов и антикоагулянтов не рекомендуется.
- Рекомендуется обеспечить лечение ОСН и угрожающих жизни нарушений сердечного ритма и проводимости в соответствии с Разделом 3.4 данных Рекомендаций.
- В карте вызова и сопроводительном талоне рекомендуется указать время начала ОКС, время первого медицинского контакта, временя регистрации ЭКГ, проведённое на догоспитальном этапе лечение с указанием доз препаратов, время доставки пациента в стационар. Если известно, рекомендуется указать препараты, принятые пациентом в ближайшие 24 ч, время их приема и дозы.
Приложение В. Информация для пациента
Острый коронарный синдром — период обострения ишемической болезни сердца, когда наиболее велика вероятность возникновения инфаркта миокарда и смерти. Наиболее частое проявление ишемии миокарда — ощущение боли или дискомфорта за грудиной. В случаях, когда эти ощущения недавно появились, участились или утяжелились — особенно если они стали возникать в покое, при незначительной физической нагрузке или носить затяжной характер — необходимо срочно обратиться за медицинской помощью (оптимально — вызвать бригаду Скорой медицинской помощи). В подобной ситуации важно оценить выраженность проявлений болезни, опасность осложнений и своевременно обнаружить другие заболевания, способствующие возникновению острого коронарного синдрома, усугубляющие его тяжесть или похожие на него по своим проявлениям. Все это может сделать только врач. Если наличие острого коронарного синдрома подтвердится, врач выберет оптимальный способ лечения, который наряду с лекарственными препаратами в виде таблеток, инъекций и инфузий может включать рентгеновское исследование сосудов сердца, откладывать которое во многих случаях нельзя. По результатам этого исследования станет ясно, есть ли необходимость в восстановлении проходимости коронарных сосудов, и, если есть — как и насколько срочно это надо делать.
Важно понимать, что в большинстве случаев острый коронарный синдром — проявление прогрессирования атеросклероза, который развивается во всех артериях человека. Поэтому после выписки повышенная угроза повторного возникновения осложнений, связанных с нарушением целостности атеросклеротических бляшек, приводящим к тромбозу, будет сохраняться долго. Поэтому очень важно не прекращать лечение, начатое в стационаре, и сосредоточить усилия на устранении факторов, способствующих прогрессированию атеросклероза (бросить курить, обеспечить регулярную физическую активность, соблюдение диеты, разработанной для таких случаев, избавиться от избыточной массы тела, поддерживать низкие значения холестерина в крови, обеспечить стойкую нормализацию артериального давления при наличии артериальной гипертонии, поддерживать нормальный уровень сахара в крови при наличии сахарного диабета). Преждевременное прекращение приема некоторых препаратов существенно увеличивает риск возникновения инфаркта миокарда и смерти от ишемической болезни сердца, поэтому нельзя этого делать, не посоветовавшись с врачом.
Приложение Г. Шкалы оценки, вопросники и другие оценочные инструменты состояния пациента, приведенные в клинических рекомендациях
Приложение Г1. Оценка риска неблагоприятного исхода при ОКСбпST с использованием шкалы GRACE
Оригинальное название: GRACE (The Global Registry of Acute Coronary Events) risk score
Источник: Granger CB, Goldberg RJ, Dabbous O, et al.; Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345-53. doi:10.1001/archinte.163.19.2345.
Тип (подчеркнуть): шкала оценки, индекс, вопросник, другое (уточнить).
Назначение: стандартизированный способ определения уровня риска смерти в стационаре у пациентов с ОКСбпST на основании перечня критериев, имеющихся на момент поступления в стационар.
Содержание:
|
Фактор риска |
Число баллов |
|
Возраст (годы) |
|
|
≤30 |
0 |
|
30-39 |
8 |
|
40-49 |
25 |
|
50-59 |
41 |
|
60-69 |
58 |
|
70-79 |
75 |
|
80-89 |
91 |
|
≥90 |
100 |
|
ЧСС (уд./мин) |
|
|
≤50 |
0 |
|
50-69 |
3 |
|
70-89 |
9 |
|
90-109 |
15 |
|
110-149 |
24 |
|
150-199 |
38 |
|
≥200 |
46 |
|
Систолическое АД (мм рт.ст.) |
|
|
≤80 |
58 |
|
80-99 |
53 |
|
100-119 |
43 |
|
120-139 |
34 |
|
140-159 |
24 |
|
160-199 |
10 |
|
≥200 |
0 |
|
Класс по Killip |
|
|
I |
0 |
|
II |
20 |
|
III |
39 |
|
IV |
59 |
|
Уровень креатинина в крови (мг/дл) |
|
|
0-0,39 |
1 |
|
0,40-0,79 |
4 |
|
0,80-1,19 |
7 |
|
1,20-1,59 |
10 |
|
1,60-1,99 |
13 |
|
2,0-3,99 |
21 |
|
≥4,0 |
28 |
|
Фактор риска |
Число баллов |
|
Другие факторы |
|
|
Остановка сердца при поступлении |
39 |
|
Смещения сегмента ST, инверсия зубца T |
28 |
|
Повышенный уровень маркеров некроза миокарда в крови* |
14 |
Ключ (интерпретация):
|
Риск смерти в стационаре |
Сумма баллов |
|
Низкий (<1%) |
≤108 |
|
Умеренный (1-3%) |
109-140 |
|
Высокий (>3%) |
≥141 |
Пояснения: * — при создании данной шкалы использовали сердечный тропонин "обычной" чувствительности.
Другие варианты шкалы GRACE 1.0 для оценки отдаленного прогноза заболевания, а также суммы случаев смерти и ИМ представлены в Интернет по адресу: https://www.outcomes-umassmed.org/grace/acs_risk/acs_risk_content.html [121][125-131].
Усовершенствованный вариант шкалы GRACE (GRACЕ 2.0) представлен в Интернет по адресу: https://www.outcomes-umassmed.org/grace/acs_risk2/index.html [131].
Усовершенствованный вариант шкалы GRACE (GRACЕ 3.0) представлен в Интернет по адресу: https://www.grace-3.com [134].
Сокращения: АД — артериальное давление, ЧСС — частота сердечных сокращений.
Приложение Г2. Шкала определения высокого риска кровотечений Консорциумa академических исследований при планирующемся/выполненном ЧКВ и/или у пациентов, нуждающихся в двойной антитромбоцитарной терапии
Оригинальное название: Academic Research Consortium for High Bleeding Risk (ARC-HBR)
Источник: Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632-53. doi:10.1093/eurheartj/ehz372 [135].
Тип (подчеркнуть): шкала оценки, индекс, вопросник, другое (уточнить).
Назначение: стандартизированный способ выявления пациентов с высоким риском кровотечений, которым планируется или которые перенесли ЧКВ и/или которым требуется двойная антитромбоцитарная терапия.
Содержание:
|
Большие критерии |
Малые критерии |
|
• Ожидаемый длительный прием перорального антикоагулянта • Тяжелая или терминальная ХБП (рСКФ <30 мл/мин/1,72 м²) • Гемоглобин <110 г/л • Спонтанное кровотечение, потребовавшее госпитализации и/или гемотрансфузии, в предшествующие 6 мес. или любой давности в случае рецидивирующего кровотечения • Умеренная или тяжелая тромбоцитопения (<100,0×10⁹/л) • Хронический геморрагический диатез • Цирроз печени с портальной гипертензией • Активное онкологическое заболевание (за исключением немеланомного рака кожи) в предшествующие 12 мес. • Перенесенное спонтанное внутричерепное кровотечение любой давности • Травматическое внутричерепное кровотечение в предшествующие 12 мес. • Указания на наличие артериовенозной мальформации головного мозга • Тяжелый или умеренной тяжести ишемический инсульт в предшествующие 6 мес. • Недавнее большое хирургическое вмешательство или серьезная травма в предшествующие 30 дней • Неотложное большое хирургическое вмешательство у пациента, получающего двойную антитромбоцитарную терапию |
• Возраст ≥75 лет • Умеренная ХБП (рСКФ 30-59 мл/мин/1,72 м²) • Гемоглобин 110-129 г/л для мужчин и 110-119 г/л для женщин • Спонтанное кровотечение, потребовавшее госпитализации и/или гемотрансфузии, в предшествующие 12 мес., не соответствующее большому критерию • Длительный пероральный прием нестероидных противовоспалительных препаратов или глюкокортикоидов • Любой ишемический инсульт независимо от его давности, не отвечающий большому критерию |
Ключ (интерпретация): о высоком риске кровотечений свидетельствует наличие как минимум одного большого или двух малых критериев.
Сокращения: рСКФ — расчетная скорость клубочковой фильтрации, ХБП — хроническая болезнь почек, ЧКВ — чрескожное коронарное вмешательство.
Приложение Г3. Оценки риска большого кровотечения у больных ОКСбпST в период госпитализации (шкала CRUSADE)
Оригинальное название: CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) bleeding score
Источник:
- Hoekstra JW, Pollack CV Jr, Roe MT, et al. Improving the care of patients with non-ST-elevation acute coronary syndromes in the emergency department: the CRUSADE initiative. Acad Emerg Med. 2002;9(11):1146-55. doi:10.1111/j.1553-2712.2002.tb01569.x.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119(14):1873-82. doi:10.1161/CIRCULATIONAHA.108.828541.
Тип (подчеркнуть): шкала оценки, индекс, вопросник, другое (уточнить).
Назначение: стандартизированный способ оценки риска большого кровотечения у больных ОКСбпST в период госпитализации.
Содержание:
|
Фактор риска |
Число баллов |
|
ЧСС (уд./мин) |
|
|
≤70 |
0 |
|
71-80 |
1 |
|
81-90 |
3 |
|
91-100 |
6 |
|
101-110 |
8 |
|
111-120 |
10 |
|
>120 |
11 |
|
Систолическое АД (мм рт.ст.) |
|
|
≤90 |
10 |
|
91-100 |
8 |
|
101-120 |
5 |
|
121-180 |
1 |
|
181-200 |
3 |
|
≥201 |
5 |
|
Гематокрит (%) |
|
|
≤31,0 |
9 |
|
31,0-33,9 |
7 |
|
34,0-36,9 |
3 |
|
37,0-39,9 |
2 |
|
≥40,0 |
0 |
|
Клиренс креатинина (мл/мин) |
|
|
≤15 |
39 |
|
>15-30 |
35 |
|
>30-60 |
28 |
|
>60-90 |
17 |
|
>90-120 |
7 |
|
>120 |
0 |
|
Другие факторы |
|
|
Женский пол |
8 |
|
Сердечная недостаточность |
7 |
|
Другое сосудистое заболевание |
6 |
|
СД |
6 |
Ключ (интерпретация):
|
Риск большого кровотечения в стационаре |
Сумма баллов |
|
Очень низкий (3,1%) |
≤20 |
|
Низкий (5,5%) |
21-30 |
|
Умеренный (8,6%) |
31-40 |
|
Высокий (11,9%) |
41-50 |
|
Очень высокий (19,5%) |
>50 |
Сокращения: АД — артериальное давление, СД — сахарный диабет, ЧСС — частота сердечных сокращений.
Приложение Г4. Прогнозирование риска кровотечений с целью определения продолжительности двойной антитромбоцитарной терапии у пациентов, имеющих к ней показания (шкала PRECISE-DAPT)
Оригинальное название: Predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score
Источник: Costa F, van Klaveren D, James S, et al.; PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-34.
Тип (подчеркнуть): шкала оценки, индекс, вопросник, другое (уточнить).
Назначение: Шкала PRECISE-DAPT была разработана для прогнозирования риска кровотечений у пациентов, имеющих показания к двойной антитромбоцитарной терапии (аспирин плюс ингибитор рецепторов P2Y12 тромбоцитов), для определения ее продолжительности.
Содержание:
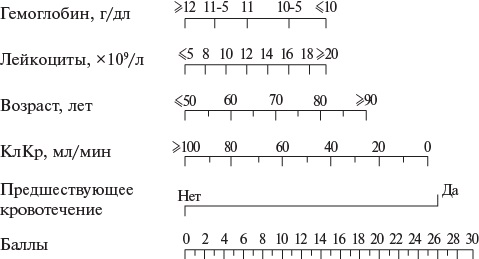
Ключ (интерпретация): на шкале каждого критерия определяется точка, соответствующая уровню данного фактора риска у пациента. От точки вниз на шкалу "баллы" проводится перпендикуляр. Его пересечение со шкалой "баллы" определяет силу данного критерия в баллах. Далее баллы суммируются. Сумма баллов может варьировать от 0 до 100.
Сумма баллов ≥25 — рекомендуется кратковременная двойная антитромбоцитарная терапия.
Сумма баллов <25 — рекомендуется стандартная/пролонгированная двойная антитромбоцитарная терапия.
В электронном формате представлена по адресу: http://www.precisedaptscore.com/predapt/webcalculator.html.
Сокращение: КлКр — клиренс креатинина.
Приложение Г5. Определение риска значимых кровотечений у больных с ОКС с использованием шкалы ОРАКУЛ, разработанной на отечественной популяции пациентов
Оригинальное название: шкала ОРАКУЛ
Источник: Бражник В. А., Минушкина Л. О., Гулиев Р. Р. и др. Факторы риска кровотечений у больных с острым коронарным синдромом: данные наблюдательного исследования ОРАКУЛ II. Российский кардиологический журнал. 2019;24:7-16.
Тип (подчеркнуть): шкала оценки, индекс, вопросник, другое (уточнить).
Назначение: Шкала Оракул была разработана на отечественной популяции пациентов для оценки риска кровотечений, требующих любого медицинского вмешательства, у пациентов, перенесших ОКС, в течение последующих 12 мес.
Содержание:
|
Фактор риска |
Число баллов |
|
Возраст |
|
|
<55 лет |
0 |
|
56-65 лет |
8 |
|
66-75 лет |
16 |
|
>75 лет |
24 |
|
Гемоглобин при поступлении (г/л) |
|
|
>125 |
0 |
|
100-125 |
48 |
|
<100 |
96 |
|
Класс сердечной недостаточности по Killip при поступлении |
|
|
I |
0 |
|
II-IV |
17 |
|
Клиренс креатинина (мл/мин) |
|
|
>90 |
0 |
|
>60-89 |
6 |
|
<60 |
12 |
|
Язвенная болезнь желудка или двенадцатиперстной кишки в анамнезе |
20 |
|
Применение антикоагулянтов в сочетании с антиагрегантами после ОКС (двойная или тройная терапия) |
36 |
|
ЧКВ во время индексной госпитализации |
38 |
Ключ (интерпретация):
|
Риск значимого кровотечения |
Сумма баллов |
|
Низкий (<1,5%), |
<67 |
|
Умеренный (2,8%), |
68-107 |
|
Высокий (5,1%), |
108-133 |
|
Очень высокий (11,7%) |
>134 |
Пояснения: шкала разработана для пациентов с ОКС любого типа, в т. ч. для ОКСбпST.
Здесь под значимыми кровотечениями понимаются кровотечения 2-5 типов по шкале BARC [46], т. е. любые, требующие дополнительных медицинских действий (диагностических и/или лечебных) [611].
В электронном формате шкала представлена по адресу: https://oracul.msk.ru/calculators/index.
Сокращения: ОКС — острый коронарный синдром, ЧКВ — чрескожное коронарное вмешательство.
Список литературы
1. Howick JH. The Philosophy of Evidence-based Medicine. Wiley. p. 15. ISBN: 978-1-44434266-6.
2. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71-2.
3. Byrne RA, Rossello X, Coughlan JJ, et al.; ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38): 3720-826. doi:10.1093/eurheartj/ehad191.
4. Андреева Н. С., Реброва О. Ю., Зорин Н. А. и др. Системы оценки достоверности научных доказательств и убедительности рекомендаций: сравнительная характеристика и перспективы унификации. Медицинские технологии. Оценка и выбор. 2012;(4):10-24.
5. Thygesen K, Alpert JS, Jaffe AS, et al.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-69.
6. Vaduganathan M, Mensah GA, Turco JV, et al. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80(25):2361-71. doi:10.1016/j.jacc.2022.11.005.
7. McManus DD, Gore J, Yarzebski J, et al. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124(1):40-7.
8. CORD-3 registry workteam. Twelve months outcomes in patients with acute coronary syndrome, by the national registry RECORD-3. Russian Journal of Cardiology. 2018;(3):23-30. (In Russ.) Эрлих А. Д. от имени всех участников регистра РЕКОРД-3. 12-месячные исходы у пациентов с острым коронарным синдромом, включённых в российский регистр рекорд-3. Российский кардиологический журнал. 2018;(3):23-30. doi:10.15829/1560-4071-2018-3-23-30.
9. Fox KA, Carruthers KF, Dunbar DR, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J. 2010;31(22):2755-64. doi:10.1093/eurheartj/ehq326.
10. Jernberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163-70. doi:10.1093/eurheartj/ehu505.
11. Jollis JG. The role of national registries. Eur Heart J. 2015;36(19):1155-6. doi:10.1093/eurheartj/ehv048.
12. Amsterdam EA, Wenger N, Brindis RG, et al. 2014 AHA/ACC Guidelines for the management of patients with non-ST-elevation acute coronary syndrome: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e344-e426.
13. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/ SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187-e285. doi:10.1016/j.jacc.2021.07.053.
14. Kontos MC, de Lemos JA, Deitelzweig SB, et al. 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(20):1925-60. doi:10.1016/j.jacc.2022. 08.750.
15. Reichlin T, Twerenbold R, Reiter M, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med. 2012;125:1205-13.
16. Сhapman AR, Lee KK, McAllister DA, et al. Association of High-Sensitivity Cardiac Troponin I Concentration With Cardiac Outcomes in Patients With Suspected Acute Coronary Syndrome. JAMA. 2017;318:1913-24.
17. Mueller C, Giannitsis E, Mockel M, et al., Biomarker Study Group of the ESC ACCA. Rapid rule out of acute myocardial infarction: novel biomarker-based strategies. Eur Heart J Acute Cardiovasc Care. 2017;6:218-22.
18. Pickering JW, Than MP, Cullen L, et al. Rapid Rule-out of Acute Myocardial Infarction With a Single High-Sensitivity Cardiac Troponin T Measurement Below the Limit of Detection: A Collaborative Meta-analysis. Ann Intern Med. 2017;166:715-3724.
19. Miller-Hodges E, Anand A, Shah ASV, et al. High-Sensitivity Cardiac Troponin and the Risk Stratification of Patients With Renal Impairment Presenting With Suspected Acute Coronary Syndrome. Circulation. 2018;137:425-35.
20. Camaro C, Aarts, GWA, Abang EMM, et al. Rule-out of non-ST-segment elevation acute coronary syndrome by a single, pre-hospital troponin measurement: a randomized trial. European Heart Journal. 2023;44(19):1705-14. doi:10.1093/eurheartj/ehad056.
21. Giannitsis E, Becker M, Kurz K, et al. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clinical chemistry. 2010;56:642-50.
22. Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136-45.
23. Shah ASV, Anand A, Strachan FE. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet. 2018;392:919-28.
24. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-8.
25. Reichlin T, Twerenbold R, Maushart C, et al. Risk stratification in patients with unstable angina using absolute serial changes of 3 high-sensitive troponin assays. Am Heart J. 2013;165:371-8.
26. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386: 2481-8.
27. Nestelberger T, Wildi K, Boeddinghaus J, et al. Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0h/1h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol. 2016;207:238-45.
28. Neumann JT, Sorensen NA, Schwemer T, et al. Diagnosis of Myocardial Infarction Using a High-Sensitivity Troponin I 1-Hour Algorithm. JAMA Cardiol. 2016;1:397-404.
29. Boeddinghaus J, Reichlin T, Cullen L, et al. Two-Hour Algorithm for Triage toward Rule-Out and Rule-In of Acute Myocardial Infarction by Use of High-Sensitivity Cardiac Troponin I. Clin Chem. 2016;62:494-504.
30. Mueller C, Giannitsis E, Christ M, et al., Investigators T-A. Multicenter Evaluation of a 0-Hour/1-Hour Algorithm in the Diagnosis of Myocardial Infarction With High-Sensitivity Cardiac Troponin T. Ann Emerg Med. 2016;68:76-87.
31. Chapman AR, Anand A, Boeddinghaus J, et al. Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation. 2017;135:1586-96.
32. Boeddinghaus J, Nestelberger T, Twerenbold R, et al. Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin I. Circulation. 2017;135:1597-611.
33. Wildi K, Cullen L, Twerenbold R, et al. Direct Comparison of 2 Rule-Out Strategies for Acute Myocardial Infarction: 2-h Accelerated Diagnostic Protocol vs 2-h Algorithm. Clin Chem. 2017;63:1227-36.
34. Boeddinghaus J, Nestelberger T, Twerenbold R, et al., Investigators T-A. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur Heart J. 2018;39:3780-94.
35. Boeddinghaus J, Twerenbold R, Nestelberger T, et al., Investigators A. Clinical Validation of a Novel High-Sensitivity Cardiac Troponin I Assay for Early Diagnosis of Acute Myocardial Infarction. Clin Chem. 2018;64:1347-60.
36. Twerenbold R, Badertscher P, Boeddinghaus J, et al. 0/1-Hour Triage Algorithm for Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2018;137:436-51.
37. Twerenbold R, Neumann JT, Sorensen NA, et al. Prospective Validation of the 0/1-h Algorithm for Early Diagnosis of Myocardial Infarction. J Am Coll Cardiol. 2018;72:620-32.
38. Greenslade J, Cho E, Van Hise C, et al. Evaluating Rapid Rule-out of Acute Myocardial Infarction Using a High-Sensitivity Cardiac Troponin I Assay at Presentation. Clin Chem. 2018;64:820-9.
39. Neumann JT, Twerenbold R, Ojeda F, et al. Application of High-Sensitivity Troponin in Suspected Myocardial Infarction. N Engl J Med. 2019;380:2529-40.
40. Neumann JT, Sorensen NA, Rubsamen N, et al. Evaluation of a new ultra-sensitivity troponin I assay in patients with suspected myocardial infarction. Int J Cardiol. 2019;283: 35-40.
41. Chapman AR, Fujisawa T, Lee KK, et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart. 2019;105:616-22.
42. Wildi K, Nelles B, Twerenbold R, et al. Safety and efficacy of the 0 h/3 h protocol for rapid rule out of myocardial infarction. Am Heart J. 2016;181:16-25.
43. Badertscher P, Boeddinghaus J, Twerenbold R, et al.; for the APACE Investigators. Direct Comparison of the 0/1h and 0/3h Algorithms for Early Rule-Out of Acute Myocardial Infarction. Circulation. 2018;137:2536-8.
44. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome [published correction appears in Am Heart J. 2007;154(5):851]. Am Heart J. 2007;153(1):29-35. doi:10.1016/j.ahj.2006.10.004.
45. Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segmentelevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873-82.
46. Бражник В. А., Минушкина Л. О., Гулиев Р. Р. и др. Факторы риска кровотечений у больных с острым коронарным синдромом: данные наблюдательного исследования ОРАКУЛ II. Российский кардиологический журнал. 2019;24(3):7-16. doi:10.15829/1560-4071-2019-3-7-16.
47. Giraldez RR, Clare RM, Lopes RD, et al. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am Heart J. 2013;165:918-25.
48. Hao Y, Lu Q, Li T, et al. Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovascular Disorders. 2017;17:6.
49. Svensson AM, McGuire DK, Abrahamsson P, Dellborg M. Association between hyperand hypoglycaemia and 2-year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J. 2005;26:1255-61.
50. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255-323. doi:10.1093/eurheartj/ehz486. Erratum in: Eur Heart J. 2020;41(45):4317.
51. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41:111-88.
52. Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157-64. doi:10.1001/jama.2011.1967.
53. Shirakabe A, Hata N, Kobayashi N, et al. Clinical significance of acid-base balance in an emergency setting in patients with acute heart failure. J Cardiol. 2012;60(4):288-94. doi:10.1016/j.jjcc.2012.06.004.
54. Maciejewski P, Bednarz B, Chamiec T, et al. Acute coronary syndrome: potassium, magnesium and cardiac arrhythmia. Kardiol Pol. 2003;59(11):402-7.
55. Babes EE, Zaha DC, Tit DM, et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics (Basel). 2021;11(5):850. doi:10.3390/diagnostics11050850.
56. Su H, Cao Y, Chen Q, et al. The association between fibrinogen levels and severity of coronary artery disease and long-term prognosis following percutaneous coronary intervention in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1287855. doi:10.3389/fendo.2023.1287855.
57. Shi Y, Wu Y, Bian C, et al. Predictive value of plasma fibrinogen levels in patients admitted for acute coronary syndrome. Tex Heart Inst J. 2010;37(2):178-83.
58. Perelló R, Calvo M, Miró O, et al. Clinical presentation of acute coronary syndrome in HIV infected adults: a retrospective analysis of a prospectively collected cohort. Eur J Intern Med. 2011;22(5):485-8. doi:10.1016/j.ejim.2011.02.017.
59. Seecheran VK, Giddings SL, Seecheran NA. Acute coronary syndromes in patients with HIV. Coron Artery Dis. 2017;28(2):166-72. doi:10.1097/MCA.0000000000000450.
60. Žvirblytė R, Ereminienė E, Montvilaitė A, et al. Syphilitic coronary artery ostial stenosis resulting in acute myocardial infarction. Medicina (Kaunas). 2017;53(3):211-6. doi:10.1016/j.medici.2017.06.001.
61. Fu Y, Chen M, Sun H, et al. Blood group A: a risk factor for heart rupture after acute myocardial infarction. BMC Cardiovasc Disord. 2020;20(1):471. doi:10.1186/s12872020-01756-y.
62. Scudiero F, Valenti R, Marcucci R, et al. Platelet Reactivity in Hepatitis C Virus-Infected Patients on Dual Antiplatelet Therapy for Acute Coronary Syndrome. J Am Heart Assoc. 2020;9(18):e016441. doi:10.1161/JAHA.120.016441.
63. Kuo PL, Lin KC, Tang PL, et al. Contribution of Hepatitis B to Long-Term Outcome Among Patients With Acute Myocardial Infarction: A Nationwide Study. Medicine (Baltimore). 2016;95(5):e2678. doi:10.1097/MD.0000000000002678.
64. Rouan GW, Lee TH, Cook EF, et al. Clinical characteristics and outcome of acute myocardial infarction in patients with initially normal or nonspecific electrocardiograms (a report from the Multicenter Chest Pain Study). The American journal of cardiology. 1989;64:1087-92.
65. McCarthy BD, Wong JB, Selker HP. Detecting acute cardiac ischemia in the emergency department: a review of the literature. J Gen Intern Med. 1990;5:365-73.
66. Savonitto S, Ardissino D, Granger CB, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA. 1999;281:707-13.
67. Diercks DB, Peacock WF, Hiestand BC, et al. Frequency and consequences of recording an electrocardiogram >10 minutes after arrival in an emergency room in non-ST-segment elevation acute coronary syndromes (from the CRUSADE initiative). Am J Cardiol. 2006;97:437-42.
68. Zalenski RJ, Rydman RJ, Sloan EP, et al. Value of posterior and right ventricular leads in comparison to the standard 12-lead electrocardiogram in evaluation of ST-segment elevation in suspected acute myocardial infarction. Am J Cardiol. 1997;79:1579-85.
69. Matetzky S, Freimark D, Feinberg MS, et al. Acute myocardial infarction with isolated ST-segment elevation in posterior chest leads V7-9: "hidden" ST-segment elevations revealing acute posterior infarction. J Am Coll Cardiol. 1999;34:748-53.
70. Fesmire FM, Percy RF, Bardoner JB, et al. Usefulness of automated serial 12-lead ECG monitoring during the initial emergency department evaluation of patients with chest pain. Ann Emerg Med. 1998;31:3-11.
71. Drozdov DV, Makarov LM, Barkan VS, et al. Resting 12-lead electrocardiography for adults and children. 2023 Guidelines. Russian Journal of Cardiology. 2023;28(10):5631. (In Russ.) Дроздов Д. В., Макаров Л. М., Баркан В. С. и др. Регистрация электрокардиограммы покоя в 12 общепринятых отведениях взрослым и детям 2023. Методические рекомендации. Российский кардиологический журнал. 2023;28(10):5631. doi:10.15829/1560-4071-2023-5631. EDN: JAVUJL.
72. Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the councils on cardiovascular nursing, clinical cardiology, and cardiovascular disease in the young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110: 2721-46.
73. Kalarus Z, Svendsen JH, Capodanno D, et al. Cardiac arrhythmias in the emergency settings of acute coronary syndrome and revascularization: an European Heart Rhythm Association (EHRA) consensus document, endorsed by the European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Acute Cardiovascular Care Association (ACCA) Europace. 2019;21:1603-4.
74. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: Incidence, predictors, and outcomes. Circulation. 2002;106:309-12.
75. Auer J, Berent R. Early prediction of life-threatening arrhythmias in non-ST elevation myocardial infarction — does it change clinical practice? European Heart Journal: Acute Cardiovascular Care. 2015;4(1):37-40.
76. Zorzi A, Turri R, Zilio F, et al. At-admission risk stratification for in-hospital life-threatening ventricular arrhythmias and death in non-ST elevation myocardial infarction patients. Eur Heart J Acute Cardiovasc Care. 2014;3(4):304-12. doi:10.1177/2048872614528796.
77. Katritsis DG, Siontis GC, Kastrati A, et al. Optimal timing of coronary angiography and potential intervention in non-ST-elevation acute coronary syndromes. Eur Heart J. 2011;32:32-40.
78. Elgendy IY, Mahmoud AN, Wen X, Bavry AA. Meta-Analysis of Randomized Trials of LongTerm All-Cause Mortality in Patients With Non-ST-Elevation Acute Coronary Syndrome Managed With Routine Invasive Versus Selective Invasive Strategies. Am J Cardiol. 2017;119:560-4.
79. Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound-guided vs angiographyguided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155-63. doi:10.1001/jama.2015.15454.
80. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drugeluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72:3126-37. doi:10.1016/j.jacc.2018.09.013.
81. Gao XF, Ge Z, Kong XQ, et al. 3-Year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14:247-57. doi:10.1016/j.jcin.2020.10.001.
82. Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation. 2016;134:906-17. doi:10.1161/circulationaha.116.024393.
83. Kala P, Cervinka P, Jakl M, et al. OCT guidance during stent implantation in primary PCI: a randomized multicenter study with nine months of optical coherence tomography followup. Int J Cardiol. 2018;250:98-103. doi:10.1016/j.ijcard.2017.10.059.
84. Johnson TW, Räber L, di Mario C, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40:2566-84. doi:10.1093/eurheartj/ehz332.
85. Sels JW, Tonino PA, Siebert U, et al. Fractional flow reserve in unstable angina and nonST-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study. JACC Cardiovasc Interv. 2011;4:1183-9. doi:10.1016/j.jcin.2011.08.008.
86. Layland J, Oldroyd KG, Curzen N, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J. 2015;36:100-11. doi:10.1093/eurheartj/ehu338.
87. Ntalianis A, Sels JW, Davidavicius G, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274-81. doi:10.1016/j.jcin.2010.08.025.
88. Peper J, Becker LM, van Kuijk J-P, et al. Fractional Flow Reserve: Patient Selection and Perspectives. Vasc Health Risk Manag. 2021;17:817-31. doi:10.2147/VHRM.S286916.
89. Brueckmann M, Collinson P, Comaniciu D, et al. Early diagnosis of acute coronary syndrome. Eur Heart J. 2017;38:3049-55.
90. Lancellotti P, Price S, Edvardsen T, et al. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2015;4:3-5.
91. Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575-81.
92. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357: 1385-90.
93. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarctioMareev Yu V. Dzhioeva ON, Zorya OT, et al. Focus ultrasound for cardiology practice. Russian consensus document. Kardiologiia. 2021;61(11):4-23. (In Russ.) Мареев Ю. В. Джиоева О. Н., Зоря О. Т. и др. Фокусное ультразвуковое исследование в кардиологической практике. Российский консенсусный документ. Кардиология. 2021; 61(11):4-23.
94. Goldschlager R, Roth H, Solomon J, et al. Validation of a clinical decision rule: chest X-ray in patients with chest pain and possible acute coronary syndrome. Emerg Radiol. 2014;21:367-72.
95. Claeys MJ, Ahrens I, Sinnaeve P, et al. The organization of chest pain units: Position statement of the Acute Cardiovascular Care Association. Eur Heart J: Acute Cardiovasc Care. 2017;6:203-11.
96. Kim HW, Faraneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction. J Am Coll Cardiol. 2009;55:1-16.
97. Beek AM, van Rossum AC. Cardiovascular magnetic resonance imaging in patients with acute myocardial infarction. Heart. 2010;96:237-43.
98. Fardman A, Massalha E, Natanzon SS, et al. Clinical predictors of left ventricular thrombus after myocardial infarction as detected by magnetic resonance imaging. Front Cardiovasc Med. 2024;10:1275390. doi:10.3389/fcvm.2023.
99. Velangi PS, Choo C, Chen KA, et al. Long-term embolic outcomes after detection of left ventricular thrombus by late gadolinium enhancement cardiovascular magnetic resonance imaging: a matched cohort study. Circ Cardiovasc Imaging. 2019;12:e009723. doi:10.1161/circimaging.119.009723.
100. Trippi JA, Lee KS. Dobutamine stress tele-echocardiography as a clinical service in the emergency department to evaluate patients with chest pain. Echocardiography. 1999;16:179-85.
101. Amsterdam EA, Kirk JD, Diercks DB, et al. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40:251-6.
102. Bholasingh R, Cornel JH, Kamp O, et al. Prognostic value of predischarge dobutamine stress echocardiography in chest pain patients with a negative cardiac troponin T. J Am Coll Cardiol. 2003;41:596-602.
103. Gaibazzi N, Reverberi C, Badano L. Usefulness of contrast stress-echocardiography or 3458 exercise-electrocardiography to predict long-term acute coronary syndromes in patients presenting with chest pain without electrocardiographic abnormalities or 12-hour troponin levation. Am J Cardiol. 2011;107:161-7.
104. Lim SH, Anantharaman V, Sundram F, et al. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department: a randomized controlled trial. J Nucl Cardiol. 2013;20:1002-12.
105. Shah BN, Balaji G, Alhajiri A, et al. Incremental diagnostic and prognostic value of contemporary stress echocardiography in a chest pain unit: mortality and morbidity outcomes from a real-world setting. Circ Cardiovasc maging. 2013;6:202-9.
106. Nabi F, Kassi M, Muhyieddeen K, et al. Optimizing Evaluation of Patients with Low-toIntermediate-Risk Acute Chest Pain: A Randomized Study Comparing Stress Myocardial Perfusion Tomography Incorporating Stress-Only Imaging Versus Cardiac CT. J Nucl Med. 2016;57:378-84.
107. Çitaku H, Miftari R, Stubljar D, Krasniqi X. Size of Acute Myocardial Infarction Correlates with Earlier Time of Initiation of Reperfusion Therapy with Cardiac Perfusion Scintigraphy: A National Single-Center Study. Med Sci Monit Basic Res. 2021;27: e933214.
108. James O, Borges-Neto S. Scintigraphic outlook of patients and regions with myocardial necrosis at myocardial perfusion scintigraphy. J Nucl Cardiol. 2018;25(2):506-7. doi:10.1007/s12350-017-0796-0. Erratum in: J Nucl Cardiol. 2018;25(2):508. doi:10.1007/s12350-017-0861-8.
109. Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53: 1642-50.
110. Goldstein JA, Chinnaiyan KM, Abidov A, et al. Investigators C-S. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414-22.
111. Samad Z, Hakeem A, Mahmood SS, et al. A meta-analysis and systematic review of computed tomography angiography as a diagnostic triage tool for patients with chest pain presenting to the emergency department. J Nucl Cardiol. 2012;19:364-76.
112. Hoffmann U, Truong QA, Schoenfeld DA, et al., Investigators R-I. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299-308.
113. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393-403.
114. Hulten E, Pickett C, Bittencourt MS, et al. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;61:880-92.
115. Dedic A, Lubbers MM, Schaap J, et al. Coronary CT Angiography for Suspected ACS in the Era of High-Sensitivity Troponins: Randomized Multicenter Study. J Am Coll Cardiol. 2016;67:16-26.
116. Goldstein JA, Gallagher MJ, O'Neill WW, et al. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863-71.
117. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Archives of internal medicine. 2003;163:2345-53.
118. Estes NA 3rd, Salem DN. Predictive value of the electrocardiogram in acute coronary syndromes. JAMA. 1999;281(8):753-4. doi:10.1001/jama.281.8.753.
119. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835-42.
120. D'Ascenzo F, Biondi-Zoccai G, Moretti C, et al. TIMI, GRACE and alternative risk scores in Acute Coronary Syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemporary clinical trials. 2012;33:507-14.
121. Lippi G, Cervellin G. Assay characteristics and diagnostic improvement from contemporary to high-sensitivity troponin I immunoassays. Am J Med. 2013;126(9):e9-e10. doi:10.1016/j.amjmed.2013.02.040.
122. Haaf P, Reichlin T, Twerenbold R, et al. Risk stratification in patients with acute chest pain using three high-sensitivity cardiac troponin assays. Eur Heart J. 2014;35:365-75.
123. Ohman EM, Granger CB, Harrington RA, Lee KL. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876-8. doi:10.1001/ jama.284.7.876.
124. Stracke S, Dörr O, Heidt MC, et al. GRACE risk score as predictor of in-hospital mortality in patients with chest pain. Clin Res Cardiol. 2010;99(10):627-31. doi:10.1007/s00392010-0160-8.
125. Yan AT, Yan RT, Tan M, et al.; Canadian ACS Registry Investigators. ST-segment depression in non-ST elevation acute coronary syndromes: quantitative analysis may not provide incremental prognostic value beyond comprehensive risk stratification. Am Heart J. 2006;152(2):270-6. doi:10.1016/j.ahj.2005.12.003.
126. Eagle KA, Lim MJ, Dabbous OH, et al.; Investigators G. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727-33.
127. Meune C, Drexler B, Haaf P, et al. The GRACE score's performance in predicting in-hospital and 1-year outcome in the era of high-sensitivity cardiac troponin assays and B-type natriuretic peptide. Heart. 2011;97:1479-83.
128. Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333:1091.
129. Fox KA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. 2014;4:e004425.
130. Simms AD, Reynolds S, Pieper K, et al. Evaluation of the NICE mini-GRACE risk scores for acute myocardial infarction using the Myocardial Ischaemia National Audit Project (MINAP) 2003-2009: National Institute for Cardiovascular Outcomes Research (NICOR). Heart. 2013;99:35-40.
131. Abu-Assi E, Raposeiras-Roubin S, Lear P, et al. Comparing the predictive validity of three contemporary bleeding risk scores in acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2012;1:222-31.
132. Costa F, van Klaveren D, James S, et al.; PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389: 1025-34.
133. Wenzl FA, Kraler S, Ambler G, et al. Sex-specific evaluation and redevelopment of the GRACE score in non-ST-segment elevation acute coronary syndromes in populations from the UK and Switzerland: a multinational analysis with external cohort validation. Lancet. 2022;400:744-56. doi:10.1016/S0140-6736(22)01483-0.
134. Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632-53. doi:10.1093/eurheartj/ehz372.
135. Минушкина Л. О., Эрлих А. Д., Бражник В. А., Затейщиков Д. А. Внешняя валидация шкалы риска кровотечений ОРАКУЛ с помощью базы данных регистра РЕКОРД 3. Кардиология. 2019;(12):5-10. doi:10.18087/cardio.2019.12.n677.
136. Бражник В. А., Минушкина Л. О., Аверкова А. О. и др. Шкалы риска кровотечений у больных с острым коронарным синдромом: место шкалы ОРАКУЛ. Кардиоваскулярная терапия и профилактика. 2020;19(5):2333. doi:10.15829/1728-8800-2020-2333.
137. Бражник В. А., Минушкина Л. О., Эрлих А. Д. и др. Использование шкалы ОРАКУЛ для оценки геморрагического риска у пациентов с острым коронарным синдромом и фибрилляцией предсердий. Рациональная Фармакотерапия в Кардиологии. 2021;17(1):11-5. doi:10.20996/1819-6446-2021-01-01.
138. Kolte D, Khera S, Dabhadkar KC, et al. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non-ST-elevation myocardial infarction. Am J Cardiol. 2016;117:1-9.
139. Mehta SR, Granger CB, Boden WE, et al.; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165-75.
140. Biondi-Zoccai G, Abbate A, Sangiorgi G. It's never too early! Evidence for rushing your acute coronary patients to the cath lab. Eur Heart J. 2011;32(1):13-5. doi:10.1093/eurheartj/ehq346.
141. Navarese EP, Gurbel PA, Andreotti F, et al. Optimal timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes: a systematic review and metaanalysis. Ann Intern Med. 2013;158:261-70.
142. Milasinovic D, Milosevic A, Marinkovic J, et al. Timing of invasive strategy in NSTE-ACS patients and effect on clinical outcomes: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. 2015;241:48-54.
143. Bonello L, Laine M, Puymirat E, et al. Timing of Coronary Invasive Strategy in Non-STSegment Elevation Acute Coronary Syndromes and Clinical Outcomes: An Updated MetaAnalysis. JACC Cardiovasc Interv. 2016;9:2267-76.
144. Jobs A, Mehta SR, Montalescot G, et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: A meta-analysis of randomised trials. Lancet. 2017;390:737-46.
145. Kofoed KF, Kelbaek H, Hansen PR, et al. Early Versus Standard Care Invasive Examination and Treatment of Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. Circulation. 2018;138:2741-50.
146. Bavry AA, Kumbhani DJ, Rassi AN, et al. Benefit of early invasive therapy in acute coronary syndromes: A meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006;48:1319-25.
147. Fox KA, Clayton TC, Damman P, et al., FIR Collaboration. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol. 2010; 55:2435-45.
148. Neumann FJ, Sousa-Uva M, Ahlsson A, et al.; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
149. Lemkes JS, Janssens GN, van der Hoeven NW, et al. Timing of revascularization in patients with transient ST-segment elevation myocardial infarction: a randomized clinical trial. Eur Heart J. 2019;40:283-91. doi:10.1093/eurheartj/ehy651.
150. Butt JH, Kofoed KF, Kelbæk H, et al. Importance of risk assessment in timing of invasive coronary evaluation and treatment of patients with non-ST-segment-elevation acute coronary syndrome: insights from the VERDICT trial. J Am Heart Assoc. 2021;10:e022333. doi:10.1161/jaha.121.022333.
151. Barbarawi M, Kheiri B, Zayed Y, et al. Meta-analysis of optimal timing of coronary intervention in non-ST-elevation acute coronary syndrome. Catheter Cardiovasc Interv. 2020;95:185-93. doi:10.1002/ccd.28280.
152. Fanning JP, Nyong J, Scott IA, et al. Routine invasive strategies versus selective invasive strategies for unstable angina and non-ST elevation myocardial infarction in the stent era. Cochrane Database Syst Rev. 2016;2016(5):CD004815. doi:10.1002/14651858.CD004815.
153. Kite TA, Kurmani SA, Bountziouka V, et al. Timing of invasive strategy in non-ST-elevation acute coronary syndrome: a meta-analysis of randomized controlled trials. Eur Heart J. 2022;43(33):3148-61. doi:10.1093/eurheartj/ehac213.
154. O'Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA. 2008;300:71-80. doi:10.1001/jama.300.1.71.
155. Mehta SR, Cannon CP, Fox KAA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005;293:2908-17. doi:10.1001/jama.293.23.2908.
156. Sanchis J, Bodí V, Núñez J, Núñez E. Conservative, true selective invasive, and routine invasive strategies in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2010;56(19):1609; author reply 1609-10. doi:10.1016/j.jacc.2010.06.032.
157. Díez-Villanueva P, Jiménez-Méndez C, Cepas-Guillén P, et al. Current Management of Non-ST-Segment Elevation Acute Coronary Syndrome. Biomedicines. 2024;12(8):1736. doi:10.3390/biomedicines12081736.
158. Collet JP, Thiele H, Barbato E, et al.; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-367. doi:10.1093/ eurheartj/ehaa575. Erratum in: Eur Heart J. 2021;42(19):1908. Erratum in: Eur Heart J. 2021;42(19):1925.
159. Morici N, De Servi S, Toso A, et al. Renal dysfunction, coronary revascularization and mortality among elderly patients with non ST elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2015;4(5):453-60. doi:10.1177/2048872614557221.
160. Nyman I, Wallentin L, Areskog M, et al. Risk stratification by early exercise testing after an episode of unstable coronary artery disease. The RISC study group. Int J Cardiol. 1993;39:131-42.
161. Siontis GC, Mavridis D, Greenwood JP, et al. Outcomes of non-invasive diagnostic modalities for the detection of coronary artery disease: network meta-analysis of diagnostic randomised controlled trials. BMJ. 2018;360:k504.
162. Sardella G, Lucisano L, Garbo R, et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016;67:264-72. doi:10.1016/j.jacc.2015.10.082.
163. Siebert VR, Borgaonkar S, Jia X, et al. Meta-analysis comparing multivessel versus culprit coronary arterial revascularization for patients with non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2019;124:1501-11. doi:10.1016/j.amjcard.2019.07.071.
164. Valgimigli M, Gagnor A, Calabro P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465-76.
165. Nardin M, Verdoia M, Barbieri L, et al. Radial vs Femoral Approach in Acute Coronary Syndromes: A Meta-Analysis of Randomized Trials. Curr Vasc Pharmacol. 2017;16:79-92.
166. Nordmann AJ, Hengstler P, Leimenstoll BM, et al. Clinical outcomes of stents versus balloon angioplasty in non-acute coronary artery disease: A meta-analysis of randomized controlled trials. Eur Heart J. 2004;25:69-80.
167. Moses JW, Mehran R, Nikolsky E, et al. Outcomes with the paclitaxel-eluting stent in patients with acute coronary syndromes: analysis from the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1165-71.
168. Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206.
169. Greenhalgh J, Hockenhull J, Rao N, et al. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev. 2010;5:CD004587.
170. Raber L, Kelbaek H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-87.
171. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625.
172. Valgimigli M, Patialiakas A, Thury A, et al.; ZEUS Investigators. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805-15.
173. Garot P, Morice MC, Tresukosol D, et al.; LEADERS FREE Investigators. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. J Am Coll Cardiol. 2017;69:162-71.
174. Darmoch F, Alraies MC, Al-Khadra Y, et al. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e013678. doi:10.1161/jaha.119.013678.
175. Jia H, Dai J, He L, et al. EROSION III: a multicenter RCT of OCT-guided reperfusion in STEMI with early infarct artery patency. JACC Cardiovasc Interv. 2022;15:846-56. doi:10.1016/j.jcin.2022.01.298.
176. Kim Y, Bae S, Johnson TW, et al.; KAMIR‐NIH (Korea Acute Myocardial Infarction Registry‐National Institutes of Health) Investigators [Link]. Role of Intravascular UltrasoundGuided Percutaneous Coronary Intervention in Optimizing Outcomes in Acute Myocardial Infarction. J Am Heart Assoc. 2022;11(5):e023481. doi:10.1161/JAHA.121.023481.
177. Kobayashi Y, Lønborg J, Jong A, et al. Prognostic value of the residual SYNTAX score after functionally complete revascularization in ACS. J Am Coll Cardiol. 2018;72:1321-9. doi:10.1016/j.jacc.2018.06.069.
178. Lee JM, Kim HK, Park KH, et al. Fractional flow reserve versus angiography-guided strategy in acute myocardial infarction with multivesse disease: a randomized trial. Eur Heart J. 2023;44:473-84. doi:10.1093/eurheartj/ehac763.
179. Подметин П. С., Бурак Т. Я., Кочанов И. Н., Каледин А. Л. Аугментация гиперемии введением дополнительного гиперемирующего агента при пограничных значениях фракционного резерва кровотока. Эндоваскулярная хирургия. 2019;6(1):13-9. doi:10.24183/24094080-2019-6-1-13-19.
180. De Bruyne B, Pijls NH, Barbato E, et al. Intracoronary and intravenous adenosine 5'-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003;107(14):1877-83. doi:10.1161/01.CIR.0000061950.24940.88.
181. Van der Voort PH, van Hagen E, Hendrix G, et al. Comparison of intravenous adenosine to intracoronary papaverine for calculation of pressure-derived fractional flow reserve. Cathet. Cardiovasc. Diagn. 1996;39(2):120-5. doi:10.1002/(SICI)1097-0304(199610)39:23.0.CO;2-H19.
182. Nishi T, Kitahara H, Iwata Y, et al. Efficacy of combined administration of intracoronary papaverine plus intravenous adenosine 5'-triphosphate in assessment of fractional flow reserve. J. Cardiol. 2016;68(6):512-6. doi:10.1016/j.jjcc.2015.12.005.
183. Li Kam Wa ME, De Silva K, Collet C, Perera D. FLOWER-MI and the root of the problem with non-culprit revascularisation. Open heart. 2021;8(2):e001763. doi:10.1136/openhrt-2021-001763.
184. Cuculi F, De Maria GL, Meier P, et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894-904. doi:10.1016/j.jacc.2014.07.987.
185. De Bruyne B, Pijls NH, Bartunek J, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 2001;104:157-62. doi:10.1161/01.cir.104.2.157.
186. Szummer K, Lundman P, Jacobson SH, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120:851-8.
187. Aspelin P, Aubry P, Fransson SG, et al. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491-9.
188. Jo SH, Youn TJ, Koo BK, et al. Renal toxicity evaluation and comparison between Visipaque (iodixanol) and Hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol. 2006;48:924-30.
189. Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189-96.
190. Brar SS, Shen AY, Jorgensen MB, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300:1038-46.
191. Maioli M, Toso A, Leoncini M, et al. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv. 2011;4:456-62.
192. Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrastinduced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, openlabel, non-inferiority trial. Lancet. 2017;389:1312-22.
193. Giacoppo D, Gargiulo G, Buccheri S, et al. Preventive Strategies for Contrast-Induced Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Procedures: Evidence From a Hierarchical Bayesian Network Meta-Analysis of 124 Trials and 28240 Patients. Circ Cardiovasc Interv. 2017;10. doi:10.1161/CIRCINTERVENTIONS.116.004383.
194. Tweet MS, Eleid MF, Best PJM, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777-86. doi:10.1161/circinterventions.114.001659.
195. Hayes SN, Tweet MS, Adlam D, et al. Spontaneous coronary artery dissection: JACC Stateof-the-Art Review. J Am Coll Cardiol. 2020;76:961-84. doi:10.1016/j.jacc.2020.05.084.
196. Adlam D, Alfonso F, Maas A, Vrints C. European Society of Cardiology, Acute Cardiovascular Care Association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-68. doi:10.1093/eurheartj/ehy080.
197. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999; 341:625-34. doi:10.1056/NEJM199908263410901.
198. Thiele H, Akin I, Sandri M, et al., Investigators C-S. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N Engl J Med. 2018;379:1699-710.
199. Lemkes JS, Janssens GN, van der Hoeven NW, et al. Coronary angiography after cardiac arrest without ST segment elevation: one-year outcomes of the COACT randomized clinical trial. JAMA Cardiol. 2020;5:1358-65. doi:10.1001/jamacardio.2020.3670.
200. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41: 3504-20. doi:10.1093/eurheartj/ehaa503.
201. Pathik B, Raman B, Mohd Amin NH, et al. Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1146-52. doi:10.1093/ehjci/jev289.
202. Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143:624-40. doi:10.1161/circulationaha.120.052008.
203. Meine TJ, Roe MT, Chen AY, et al. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149:1043-9.
204. Iakobishvili Z, Cohen E, Garty M, et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care. 2011;13:76-80.
205. Duarte GS, Nunes-Ferreira A, Rodrigues FB, et al. Morphine in acute coronary syndrome: systematic review and meta-analysis. BMJ Open. 2019;9:e025232.
206. Ghadban R, Enezate T, Payne J, et al. The safety of morphine use in acute coronary syndrome: a meta-analysis. Heart Asia. 2019;11:e011142.
207. Parodi G, Bellandi B, Xanthopoulou I, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2015; 8:e001593. doi:10.1161/circinterventions.114.001593.
208. Saad M, Meyer-Saraei R, de Waha-Thiele S, et al. Impact of morphine treatment with and without metoclopramide coadministration on ticagrelor-induced platelet inhibition in acute myocardial infarction: the randomized MonAMI trial. Circulation. 2020;141:1354-6. doi:10.1161/circulationaha.119.042816.
209. Murphy B, Le Grande M, Alvarenga M, et al. Anxiety and depression after a cardiac event: prevalence and predictors. Front Psychol. 2019;10:3010. doi:10.3389/fpsyg. 2019.03010.
210. Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2011;2011(9):CD008012. doi:10.1002/14651858.CD008012.pub3.
211. Moser DK. "The rust of life": impact of anxiety on cardiac patients. Am J Crit Care. 2007;16:361-9. doi:10.4037/ajcc2007.16.4.361.
212. Farquhar H, Weatherall M, Wijesinghe M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158:371-7.
213. Moradkhan R, Sinoway LI. Revisiting the role of oxygen therapy in cardiac patients. JACC. 2010;56:1013-6.
214. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143-50. doi:10.1161/circulationaha.114.014494.
215. Cabello JB, Burls A, Emparanza JI, et al. Oxygen therapy for acute myocardial infarction. Cochrane Database Syst Rev. 2016;12:CD007160.
216. Hofmann R, James SK, Jernberg T, et al.; for the DETO2X–SWEDEHEART Investigators. Oxygen Therapy in Suspected Acute Myocardial Infarction. E Engl J Med. 2017;377:12409. doi:10.1056/NEJMoa1706222.
217. Lewis HD Jr, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration cooperative study. N Engl J Med. 1983;309:396-403.
218. Cairns JA, Gent M, Singer J, et al. Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. N Engl J Med. 1985;313:1369-75.
219. Theroux P, Ouimet H, McCans J, et al. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988;319:1105-11.
220. The RISC group. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet. 1990;336:827-30.
221. Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86.
222. Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-60.
223. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-39.
224. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345: 494-502.
225. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-15.
226. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-57.
227. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527-33.
228. Mehta SR, Tanguay JF, Eikelboom JW, et al.; for CURRENT-OASIS Trial Investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-43.
229. Mega JL, Braunwald E, Wiviott SD, et al.; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19.
230. Eikelboom JW, Anand SS, Malmberg K, et al. Unfractionated heparin and low-molecularweight heparin in acute coronary syndrome without ST elevation: A meta-analysis. Lancet. 2000;355:1936-42.
231. Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464-76.
232. Steg PG, Jolly SS, Mehta SR, et al. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339-49.
233. Silvain J, Beygui F, Barthelemy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
234. Cohen M, Mahaffey KW, Pieper K, et al.; on behalf of the SYNERGY Trial Investigators. A Subgroup Analysis of the Impact of Prerandomization Antithrombin Therapy on Outcomes in the SYNERGY Trial Enoxaparin Versus Unfractionated Heparin in Non-STSegment Elevation Acute Coronary Syndromes. JACC. 2006;48:1346-54.
235. Mehta SR, Steg PG, Granger CB, et al. Randomized, blinded trial comparing fondaparinux with unfractionated heparin in patients undergoing contemporary percutaneous coronary intervention: Arixtra Study in Percutaneous coronary Intervention: a Randomized Evaluation (ASPIRE) pilot trial. Circulation. 2005;111:1390-7.
236. Cassese S, Byrne RA, Laugwitz KL, et al. Bivalirudin versus heparin in patients treated with percutaneous coronary intervention: a meta-analysis of randomised trials. EuroIntervention. 2015;11:196-203.
237. Zhang S, Gao W, Li H, et al. Efficacy and safety of bivalirudin versus heparin in patients undergoing percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Int J Cardiol. 2016;209:87-95.
238. Erlinge D, Omerovic E, Frobert O, et al. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med. 2017;377:1132-42.
239. Nuhrenberg TG, Hochholzer W, Mashayekhi K, et al. Efficacy and safety of bivalirudin for percutaneous coronary intervention in acute coronary syndromes: a meta-analysis of randomized-controlled trials. Clin Res Cardiol. 2018;107:807-15.
240. Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013;62:981-9.
241. Fiedler KA, Maeng M, Mehilli J, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: The ISAR-TRIPLE trial. J Am Coll Cardiol. 2015;65:1619-29.
242. Dewilde WJ, Janssen PW, Kelder JC, et al. Uninterrupted oral anticoagulation versus bridging in patients with long-term oral anticoagulation during percutaneous coronary intervention: subgroup analysis from the WOEST trial. EuroIntervention. 2015;11:381-90.
243. Dewilde WJ, Oirbans T, Verheugt FW, et al.; WOEST Study Investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013;381:1107-15.
244. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423-34.
245. Cannon CP, Bhatt DL, Oldgren J, et al.; RE-DUAL Steering Committee PCI and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-24.
246. Lopes RD, Heizer G, Aronson R, et al.; for the AUGUSTUS Investigators. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation NEJM. 2019; 380:1509-24.
247. Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40;3757-67.
248. Lopes RD, Hong H, Harskamp RE, et al. Optimal Antithrombotic Regimes for Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. An Updated Network Meta-analysis. JAMA Cardiol. 2020. doi:10.1001/jamacardio.2019.6175.
249. Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012; 126:1185-93.
250. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation. 2013;127:634-40.
251. Kopin D, Jones WS, Sherwood MW, et al. Percutaneous coronary intervention and antiplatelet therapy in patients with atrial fibrillation receiving apixaban or warfarin: Insights from the ARISTOTLE trial. Am Heart J. 2018;197:133-41.
252. Kuno T, Watanabe A, Shoji S, et al. Short-Term DAPT and DAPT De-Escalation Strategies for Patients With Acute Coronary Syndromes: A Systematic Review and Network Meta-Analysis. Circ Cardiovasc Interv. 2023;16(9):e013242. doi:10.1161/CIRCINTERVENTIONS.123.013242.
253. Wang W, Huang Q, Pan D, et al. The optimal duration of triple antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome or undergoing percutaneous coronary intervention: A network meta-analysis of randomized clinical trials. Int J Cardiol. 2022;357:33-8. doi:10.1016/j.ijcard.2022.03.047.
254. Kuno T, Ueyama H, Takagi H, Bangalore S. The risk of stent thrombosis of dual antithrombotic therapy for patients who require oral anticoagulant undergoing percutaneous coronary intervention: insights of a meta-analysis of randomized trials. Scand Cardiovasc J. 2022;56(1):1-3. doi:10.1080/14017431.2021.2025264.
255. Gupta R, Malik AH, Gupta R, et al. Dual Versus Triple Therapy in Patients with Acute Coronary Syndrome and an Anticoagulation Indication: A Systematic Review with MetaAnalysis and Trial-Sequential Analysis. Cardiol Rev. 2021;29(5):245-52. doi:10.1097/CRD.0000000000000320.
256. Agarwal N, Mahmoud AN, Mojadidi MK, et al. Dual versus triple antithrombotic therapy in patients undergoing percutaneous coronary intervention-meta-analysis and metaregression. Cardiovasc Revasc Med. 2019;20(12):1134-9. doi:10.1016/j.carrev.2019.02.022.
257. Zhang J, Wang Z, Sang W, et al. Omission of aspirin in patients taking oral anticoagulation after percutaneous coronary intervention: a systematic review and meta-analysis. Coron Artery Dis. 2019;30(2):109-15. doi:10.1097/MCA.0000000000000698.
258. Shah R, Khan SA, Khan B, Latham SB. Short-term versus long-term triple antithrombotic therapy for patients with coronary stents and requiring oral anticoagulation: a metaanalysis of randomized clinical trials. Coron Artery Dis. 2019;30(2):116-23. doi:10.1097/MCA.0000000000000690.
259. Shin D, Mohanty BD, Lee ES. Dual versus triple antithrombotic therapy after percutaneous coronary intervention or acute coronary syndrome in patients with indication for anticoagulation: an updated meta-analysis. Coron Artery Dis. 2018;29(8):670-80. doi:10.1097/MCA.0000000000000660.
260. Valgimigli M, Bueno H, Byrne RA, et al. ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for CardioThoracic Surgery (EACTS). Eur Heart J. 2018;39:213-60.
261. Schü e S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524-34. doi:10.1056/NEJMoa1908973.
262. Montalescot G, Bolognese L, Dudek D, et al. Pretreatment with prasugrel in non-STsegment elevation acute coronary syndromes. N Engl J Med. 2013;369:999-1010. doi:10.1056/NEJMoa1308075.
263. Tarantini G, Mojoli M, Varbella F, et al. Timing of oral P2Y(12) inhibitor administration in patients with non-ST-segment elevation acute coronary syndrome. JACC. 2020;76:2450-9. doi:10.1016/j.jacc.2020.08.053.
264. Dworeck C, Redfors B, Angerås O, et al. Association of pretreatment with P2Y12 receptor antagonists preceding percutaneous coronary intervention in non-ST-segment elevation acute coronary syndromes with outcomes. JAMA Netw Open. 2020;3:e2018735. doi:10.1001/jamanetworkopen.2020.18735.
265. Steinhubl SR, Berger PB, Mann JT III, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411-20. doi:10.1001/jama.288.19.2411.
266. Dawson LP, Chen D, Dagan M, et al. Assessment of pretreatment with oral P2Y12 inhibitors and cardiovascular and bleeding outcomes in patients with non-ST elevation acute coronary syndromes: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2134322. doi:10.1001/jamanetworkopen.2021.34322.
267. Fox KA, Mehta SR, Peters R, et al. Benefits and Risks of the Combination of Clopidogrel and Aspirin in Patients Undergoing Surgical Revascularization for Non-ST-Elevation Acute Coronary Syndrome. The Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110:1202-8.
268. Wiviott SD, White HD, Ohman EM, et al. Prasugrel versus clopidogrel for patients with unstable angina or non-ST-segment elevation myocardial infarction with or without angiography: a secondary, prespecified analysis of the TRILOGY ACS trial. Lancet. 2013;382:605-13. doi:10.1016/s0140-6736(13)61451-8.
269. James SK, Roe MT, Cannon CP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ. 2011;342:d3527. doi:10.1136/bmj.d3527.
270. Kohli P, Wallentin L, Reyes E, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation. 2013;127(6): 673-80. doi:10.1161/CIRCULATIONAHA.112.1242487.
271. Kleiman NS. PLATO study of ticagrelor versus clopidogrel in patients with high-risk acute coronary syndromes. Curr Cardiol Rep. 2010;12(4):283-5. doi:10.1007/s11886010-0114-9.
272. Coughlan JJ, Aytekin A, Lahu S, et al. Ticagrelor or prasugrel for patients with acute coronary syndrome treated with percutaneous coronary intervention: a prespecified subgroup analysis of a randomized clinical trial. JAMA Cardiol. 2021;6:1121-9. doi:10.1001 jamacardio.2021.2228.
273. Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, openlabel, non-inferiority trial. Lancet. 2020;395: 1374-81. doi:10.1016/s0140-6736(20)30325-1.
274. Husted S, James S, Becker RC, et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes. 2012;5:680-8. doi:10.1161/circoutcomes.111.964395.
275. Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020;142:1700-8. doi:10.1161/circulationaha.120.050645.
276. Khan SU, Khan MZ, Asad ZUA, et al. Efficacy and safety of low dose rivaroxaban in patients with coronary heart disease: a systematic review and meta-analysis. J Thromb Thrombolysis. 2020;50(4):913-20. doi:10.1007/s11239-020-02114-7.
277. Stone GW, Bertrand ME, Moses JW, et al. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY timing trial. JAMA. 2007;297:591-602.
278. Giugliano RP, White JA, Bode C, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009;360:2176-90.
279. Sehested TSG, Carlson N, Hansen PW, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J. 2019;40:1963-70.
280. Bhatt DL, Cryer BL, Contant CF, et al.; for the COGENT Investigators. Clopidogrel with or without Omeprazole in Coronary Artery Disease. N Engl J Med. 2010;363:1909-17.
281. Kwok CSh, Jeevanantham V, Dawn B, Loke YK. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: Metaanalysis. Int J Cardiol. 2012;167:965-74.
282. Huang B, Huang Y, Li Y, et al. Adverse Cardiovascular Effects of Concomitant Use of Proton Pump Inhibitors and Clopidogrel in Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. Archives of Medical Research. 2012;43:212-24.
283. Melloni C, Washam JB, Jones WS, et al. Conflicting Results Between Randomized Trials and Observational Studies on the Impact of Proton Pump Inhibitors on Cardiovascular Events When Coadministered With Dual Antiplatelet Therapy. Systematic Review. Circ Cardiovasc Qual Outcomes. 2015;8:47-55.
284. O'Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-97. doi:10.1016/s0140-6736(09)61525-7.
285. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-86. doi:10.1161/circulationaha.111.032912.
286. Long M, Ye Z, Zheng J, et al. Dual anti-platelet therapy following percutaneous coronary intervention in a population of patients with thrombocytopenia at baseline: a metaanalysis. BMC Pharmacol Toxicol. 2020;21:31. doi:10.1186/s40360-020-00409-2.
287. Palmerini T, Della Riva D, Benedetto U, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11473 patients. Eur Heart J. 2017;38:1034-43. doi:10.1093/eurheartj/ehw627.
288. Hahn J-Y, Song YB, Oh J-H, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321:2428-37. doi:10.1001/jama.2019.8146.
289. Vranckx P, Valgimigli M, Jüni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomized superiority trial. Lancet. 2018;392:940-9. doi:10.1016/s0140-6736(18)31858-0.
290. Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381:2032-42. doi:10.1056/NEJMoa1908419.
291. Kim BK, Hong SJ, Cho YH, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323:2407-16. doi:10.1001/jama.2020.7580.
292. Giacoppo D, Matsuda Y, Fovino LN, et al. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with secondgeneration drug-eluting stents: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2021;42:308-19. doi:10.1093/eurheartj/ehaa739.
293. Valgimigli M, Gragnano F, Branca M, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ. 2021;373:n1332. doi:10.1136/bmj.n1332.
294. Smits PC, Frigoli E, Tijssen J, et al. Abbreviated antiplatelet therapy in patients at high bleeding risk with or without oral anticoagulant therapyafter coronary stenting: an open-label, randomized, controlled trial. Circulation. 2021;144:1196-211. doi:10.1161/circulationaha.121.056680.
295. Watanabe H, Morimoto T, Natsuaki M, et al.; STOPDAPT-2 ACS Investigators. Comparison of Clopidogrel Monotherapy After 1 to 2 Months of Dual Antiplatelet Therapy With 12 Months of Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome: The STOPDAPT-2 ACS Randomized Clinical Trial. JAMA Cardiol. 2022;7(4):407-17. doi:10.1001/jamacardio.2021.5244.
296. Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, openlabel, multicentre trial. Lancet. 2017;390:1747-57. doi:10.1016/s0140-6736(17)32155-4.
297. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-8. doi:10.1093/eurheartj/ehx175.
298. Kim CJ, Park MW, Kim MC, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. 2021;398:1305-16. doi:10.1016/s01406736(21)01445-8.
299. Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381:1621-31. doi:10.1056/NEJMoa1907096.
300. Oler A, Whooley MA, Oler J, Grady D. Adding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina. A meta-analysis. JAMA. 1996;276:811-5. doi:10.1001/jama.1996.03540100055028.
301. Ferguson JJ, Califf RM, Antman EM, et al. Enoxaparin vs unfractionated heparin in highrisk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45-54. doi:10.1001/jama.292.1.45.
302. James S. Enoxaparin for anticoagulation in patients undergoing percutaneous coronary intervention. BMJ. 2012;344:e712. doi:10.1136/bmj.e712.
303. Antman EM, Cohen M, Radley D, et al. Assessment of the treatment effect of enoxaparin for unstable angina/non-Q-wave myocardial infarction: TIMI 11B-ESSENCE metaanalysis. Circulation. 1999;100:1602-8. doi:10.1161/01.cir.100.15.1602.
304. Martin JL, Fry ETA, Sanderink G.-JCM, et al. Reliable anticoagulation with enoxaparin in patients undergoing percutaneous coronary intervention: The pharmacokinetics of enoxaparin in PCI (PEPCI) study. Cardiovasc Interv. 2004;61:163-70. doi:10.1002/ccd.10726.
305. Cohen M, Levine GN, Pieper KS, et al.; SYNERGY Trial Investigators. Enoxaparin 0.3 mg/kg IV supplement for patients transitioning to PCI after subcutaneous enoxaparin therapy for NSTE ACS: a subgroup analysis from the SYNERGY trial. Catheter Cardiovasc Interv. 2010;75:928-35. doi:10.1002/ccd.22340.
306. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383:955-62.
307. Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonistbased antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019; 394:1335-43. doi:10.1016/s0140-6736(19)31872-0.
308. Carter NJ, Plosker GL. Rivaroxaban: a review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Drugs. 2013;73(7):715-39. doi:10.1007/s40265-013-0056-9.
309. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). European Heart Journal. 2020;42:373-498.
310. Ray WA, Chung CP, Murray KT, et al. Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy With Hospitalization for Upper Gastrointestinal Tract Bleeding. JAMA. 2018;320:2221-30. doi:10.1001/jama.2018.17242.
311. Moayyedi P, Eikelboom JW, Bosch J, et al.; COMPASS Investigators. Pantoprazole to Prevent Gastroduodenal Events in Patients Receiving Rivaroxaban and/or Aspirin in a Randomized, Double-Blind, Placebo-Controlled Trial Gastroenterology. 2019;157(2): 403-12. doi:10.1053/j.gastro.2019.04.041.
312. Lee SR, Kwon S, Choi EK, et al. Proton Pump Inhibitor Co-Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulants and a Prior History of Upper Gastrointestinal Tract Bleeding. Cardiovasc Drugs Ther. 2022;36:679-89. doi:10.1007/s10557-02107170-6.
313. Ahn HJ, Lee SR, Choi EK, et al. Protective effect of proton-pump inhibitor against gastrointestinal bleeding in patients receiving oral anticoagulants: A systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88:4676-87. doi:10.1111/bcp.15478.
314. Hahn J-Y, Song YBin, Oh J-H, et al.; for the SMART-DATE investigators. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391:1274-84.
315. Mauri L, Kereiakes DJ, Yeh RW, et al.; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155-66.
316. Costa F, Adamo M, Ariotti S, et al. Impact of greater than 12-month dual antiplatelet therapy duration on mortality: Drug-specific or a class-effect? A meta-analysis. Int J Cardiol. 2015;201:179-81.
317. Hermiller JB, Krucoff MW, Kereiakes DJ, et al.; DAPT Study Investigators. Benefits and risks of extended dual antiplatelet therapy after everolimus-eluting stents. JACC Cardiovasc Interv. 2016;9:138-47.
318. Udell JA, Bonaca MP, Collet JP, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 2016;37: 390-9.
319. Bonaca MP, Bhatt DL, Cohen M, et al.; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-800.
320. Bonaca MP, Bhatt DL, Steg PG, et al. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: Insights from PEGASUS-TIMI 54. Eur Heart J. 2016;37:1133-42.
321. Eikelboom JW, Connolly SJ, Bosch JD, et al.; for the COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319-30. doi:10.1056/NEJMoa1709118.
322. Chiarito M, Sanz-Sánchez J, Cannata F, et al. Monotherapy with a P2Y(12) inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet. 2020;395:1487-95. doi:10.1016/s01406736(20)30315-9.
323. Koo BK, Kang J, Park KW, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigatorinitiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021;397:248796. doi:10.1016/s0140-6736(21)01063-1.
324. Leonardi S, Franzone A, Piccolo R, et al. Rationale and design of a prospective substudy of clinical endpoint adjudication processes within an investigator-reported randomised controlled trial in patients with coronary artery disease: the GLOBAL LEADERS Adjudication Sub-StudY (GLASSY). BMJ Open. 2019;9(3):e026053. doi:10.1136/bmjopen-2018-026053.
325. Franzone A, McFadden E, Leonardi S, et al.; GLASSY Investigators. Ticagrelor Alone Versus Dual Antiplatelet Therapy From 1 Month After Drug-Eluting Coronary Stenting. J Am Coll Cardiol. 2019;74(18):2223-34. doi:10.1016/j.jacc.2019.08.1038.
326. Borzak S, Cannon CP, Kraft PL, et al. Effects of prior aspirin and anti-ischemic therapy on outcome of patients with unstable angina. TIMI 7 Investigators. Thrombin inhibition in myocardial ischemia. Am J Cardiol. 1998;81:678-81.
327. Melandri G, Branzi A, Tartagni F, et al. Haemodynamic effects of metoprolol and intravenous nitroglycerin versus metoprolol alone in patients with acute myocardial infarction. Eur Heart J. 1987;8:592-6.
328. Zharov EI, Vertkin AL, Martynov AI, Salnikov SN. Comparative evaluation of intravenous isosorbide dinitrate and nitroglycerin in patients with acute myocardial infarction. Cardiology. 1991;79 Suppl 2:63-9. doi:10.1159/000174927.
329. Hirota Y, Saito T, Kita Y, et al. Intravenous isosorbide dinitrate infusion in the management of unstable angina pectoris refractory to conventional medical therapy. Jpn Circ J. 1987;51(6):617-23. doi:10.1253/jcj.51.617.
330. Kojima S, Matsui K, Sakamoto T, et al.; on behalf of the Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Long-term nitrate therapy after acute myocardial infarction does not improve or aggravate prognosis. Circ J. 2007;71:301-7.
331. Miyazaki S, Nonogi H, Goto Y, et al. Comparison of the therapeutic efficacy of continuous and intermittent injection of isosorbide dinitrate: a randomized study on unstable angina. Intern Med. 1995;34(9):856-62. doi:10.2169/internalmedicine.34.856.
332. Freemantle N, Cleland J, Young P, et al. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730-7.
333. Ellis K, Tcheng JE, Sapp S, et al. Mortality benefit of beta-blockade in patients with acute coronary syndromes undergoing coronary intervention: pooled results from Epic, Epilog, Capture and Rapport trials. J Interven Cardiol. 2003;16:299-305.
334. Miller CD, Roe MT, Mulgund J, et al. Impact of acute beta-blocker therapy for patients with non-ST-segment elevation myocardial infarction. Am J Med. 2007;120:685-92.
335. Chatterjee S, Chaudhuri D, Vedanthan R, et al. Early intravenxous beta-blockers in patients with acute coronary syndrome — a meta-analysis of randomized trials. Int J Cardiol. 2013;168:915-21.
336. Andersson C, Shilane D, Go AS, et al. Beta-blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64:247-52.
337. Bangalore S, Makani H, Radford M, et al. Clinical Outcomes with β-Blockers for Myocardial Infarction: A Meta-analysis of Randomized Trials. Am J Med. 2014;127:939-53.
338. Puymirat E, Riant E, Aissoui Na, et al. β-blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016; 354:i4801.
339. Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). European Heart Journal. 2022;43:3997-4126.
340. CIBIS-II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomized trial. Lancet. 1999;353:9-13.
341. MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353:2001-7.
342. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651-8.
343. Maclean E, Zheng S, Nabeebaccus A, et al. Effect of early bisoprolol administration on ventricular arrhythmia and cardiac death in patients with non-ST elevation myocardial infarction. Heart Asia. 2015;7(2):46-51. doi:10.1136/heartasia-2015-010675.
344. de Peuter OR, Lussana F, Peters RJ, et al. A systematic review of selective and nonselective beta blockers for prevention of vascular events in patients with acute coronary syndrome or heart failure. Neth J Med. 2009;67:284-94.
345. Munkhaugen J, Ruddox V, Halvorsen S, et al. BEtablocker treatment after acute myocardial infarction in revascularized patients without reduced left ventricular ejection fraction (BETAMI): rationale and design of a prospective, randomized, open, blinded end point study. Am Heart J. 2019;208:37-46.
346. Kristensen AMD, Bovin A, Zwisler AD, et al. Design and rationale of the Danish trial of beta-blocker treatment after myocardial infarction without reduced ejection fraction: study protocol for a randomized controlled trial. Trials. 2020;21:1-11.
347. Rossello X, Raposeiras-Roubin S, Latini R, et al. Rationale and design of the pragmatic clinical trial tREatment with beta-blockers after myOcardial infarction withOut reduced ejection fracTion (REBOOT). Eur Heart J Cardiovasc Pharmacother. 2022;8(3):291-301.
348. Yndigegn T, Lindahl B, Alfredsson J, et al. Design and rationale of randomized evaluation of decreased usage of beta-blockers after acute myocardial infarction (REDUCE-AMI). Eur Heart J Cardiovasc Pharmacother. 2023;9(2):192-7. doi:10.1093/ehjcvp/pvac070.
349. Safi S, Sethi NJ, Korang SK, et al. Beta-blockers in patients without heart failure after myocardial infarction. Cochrane Database Syst Rev. 2021;11(11):CD012565. doi:10.1002/14651858.
350. Wen XS, Luo R, Liu J, et al. Short-term/long-term prognosis with or without betablockers in patients without heart failure and with preserved ejection fraction after acute myocardial infarction: a multicenter retrospective cohort study. BMC Cardiovasc Disord. 2022;22(1):193. doi:10.1186/s12872-022-02631-8.
351. Won H, Suh Y, Kim GS, et al. Clinical Impact of Beta Blockers in Patients with Myocardial Infarction from the Korean National Health Insurance Database. Korean Circ J. 2020; 50(6):499-508. doi:10.4070/kcj.2019.0231.
352. Sakagami A, Soeda T, Saito Y, et al.; J-MINUET investigators. Clinical impact of betablockers at discharge on long-term clinical outcomes in patients with non-reduced ejection fraction after acute myocardial infarction. J Cardiol. 2023;81(1):83-90. doi:10.1016/j.jjcc.2022.08.002.
353. Desta L, Khedri M, Jernberg T, et al. Adherence to beta-blockers and long-term risk of heart failure and mortality after a myocardial infarction. ESC Heart Fail. 2021;8(1): 344-55. doi:10.1002/ehf2.13079.
354. Song PS, Kim M, Seong SW, et al. Heart failure with mid-range ejection fraction and the effect of β-blockers after acute myocardial infarction. Heart Vessels. 2021;36(12):184855. doi:10.1007/s00380-021-01876-1.
355. Velásquez-Rodríguez J, Bruña V, Vicent L, et al. Influence of left ventricular systolic function on the long-term benefit of beta-blockers after ST-segment elevation myocardial infarction. Rev Port Cardiol (Engl Ed). 2021;40(4):285-90. doi:10.1016/j.repc.2020.07.017.
356. Gibson RS, Boden WE, Theroux P, et al. Diltiazem and reinfarction in patients with non-Qwave myocardial infarction. Results of a double-blind, randomized, multicenter trial. N Engl J Med. 1986;315:423-9.
357. Early treatment of unstable angina in the coronary care unit: a randomised, double blind, placebo-controlled comparison of recurrent ischaemia in patients treated with nifedipine or metoprolol or both. Report of the Holland Interuniversity Nifedipine/Metoprolol Trial (HINT) Research Group. Br Heart J. 1986;56:400-13.
358. Lubsen J, Tijssen JG. Efficacy of nifedipine and metoprolol in the early treatment of unstable angina in the coronary care unit: findings from the Holland Interuniversity Nifedipine/metoprolol Trial (HINT). Am J Cardiol. 1987;60:18A-25A.
359. Held PH, Yusuf S, Furberg CD. Calcium channel blockers in acute myocardial infarction and unstable angina: an overview. BMJ. 1989;299:1187-92.
360. Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial II-DAVIT II). Am J Cardiol. 1990;66:779-85.
361. Moss AJ, Oakes D, Rubison M, et al. Effects of diltiazem on long-term outcome after acute myocardial infarction in patients with and without a history of systemic hypertension. The Multicenter Diltiazem Postinfarction Trial Research Group. Am J Cardiol. 1991;68:429-33.
362. Rengo F, Carbonin P, Pahor M, et al. A controlled trial of verapamil in patients after acute myocardial infarction: results of the calcium antagonist reinfarction Italian study (CRIS). Am J Cardiol. 1996;77:365-9.
363. Pepine CJ, Faich G, Makuch R. Verapamil use in patients with cardiovascular disease: an overview of randomized trials. Clin Cardiol. 1998;21:633-41.
364. Smith NL, Reiber GE, Psaty BM, et al. Health outcomes associated with beta-blocker and diltiazem treatment of unstable angina. J Am Coll Cardiol. 1998;32:1305-11.
365. Beltrame JF, Crea F, Kaski JC, et al.; Coronary Vasomotion Disorders International Study Group (COVADIS). The Who, What, Why, When, How and Where of Vasospastic Angina. Circ J. 2016;80:289-98.
366. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-53.
367. Fox KM; European trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebocontrolled, multicentre trial (the EUROPA study). Lancet. 2003;362:782-8.
368. ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547-59. doi:10.1056/NEJMoa0801317.
369. Bangalore S, Fakheri R, Wandel S, et al. Renin angiotensin system inhibitors for patients with stable coronary artery disease without heart failure: systematic review and metaanalysis of randomized trials. BMJ. 2017;356:j4.
370. Pfeffer MA, Braunwald E, Moye LA, et al. SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669-77.
371. Ball SG, Hall AS, Murray GD. ACE inhibition, atherosclerosis and myocardial infarction — the AIRE Study in practice. Acute Infarction Ramipril Efficacy Study. Eur Heart J. 1994;15(Suppl B):20-5.
372. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet. 1995;345:669-85.
373. Pfeffer MA, Greaves SC, Arnold JM, et al. Early versus delayed angiotensin-converting enzyme inhibition therapy in acute myocardial infarction. The healing and early afterload reducing therapy trial. Circulation. 1997;95:2643-51.
374. ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. Circulation. 1998;97:2202-12.
375. Pop D, Dădârlat-Pop A, Tomoaia R, et al. Updates on the Renin-Angiotensin-Aldosterone System and the Cardiovascular Continuum. Biomedicines. 2024;12(7):1582. doi:10.3390/biomedicines12071582.
376. Borghi C, Omboni S, Reggiardo G, et al.; on behalf of the SMILE Working Project. Efficacy of Zofenopril Compared With Placebo and Other Angiotensin-converting Enzyme Inhibitors in Patients With Acute Myocardial Infarction and Previous Cardiovascular Risk Factors: A Pooled Individual Data Analysis of 4 Randomized, Double-blind, Controlled, Prospective Studies. J Cardiovasc Pharmacol. 2017;69:48-54.
377. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893-906.
378. Weber MA. Telmisartan in high-risk cardiovascular patients. Am J Cardiol. 2010;105 (1 Suppl):36A-43A. doi:10.1016/j.amjcard.2009.10.008.
379. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan 5688 in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to 5689 angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lance. 2003;362772-6.
380. Berwanger O, Pfeffer M, Claggett D, et al. Sacubitril/valsartan versus ramipril for patients with acute myocardial infarction: win-ratio analysis of the PARADISE-MI trial. European Journal of Heart Failure. 2022. doi:10.1002/ejhf.2663.
381. Gu J, Wang Y, Wang CQ, Zhang JF. The initial timing and dosage pattern of sacubitril/ valsartan in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Eur J Intern Med. 2023:S0953-6205(23)00091-2. doi:10.1016/j.ejim.2023.03.019.
382. Jering KS, Claggett B, Pfeffer MA, et al. Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure Events after Myocardial Infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. 2021;23(6):1040-8. doi:10.1002/ejhf.2191.
383. Lin G, Chen W, Wu M, et al. The value of sacubitril/valsartan in acute anterior wall ST-segment elevation myocardial infarction before emergency Percutaneous coronary intervention. Cardiology. 2022. doi:10.1159/000527357.
384. Mehran R, Steg PhG, Pfeffer MA, et al. The Effects of Angiotensin Receptor-Neprilysin Inhibition on Major Coronary Events in Patients With Acute Myocardial Infarction: Insights From the PARADISE-MI Trial. Circulation. 2022;146:1749-57. doi:10.1161/ CIRCULATIONAHA.122.060841.
385. Pfeffer MA, Claggett B, Lewis EF, et al. Impact of Sacubitril/Valsartan Versus Ramipril on Total Heart Failure Events in the PARADISE-MI Trial. Circulation. 2022;145:87-9. doi:10.1161/CIRCULATIONAHA.121.057429.
386. Pfeffer MA, Claggett B, Lewis EF, et al. Angiotensin Receptor–Neprilysin Inhibition in Acute Myocardial Infarction. N Engl J Med. 2021;385:1845-55. doi:10.1056/ NEJMoa2104508.
387. Rezq A, Saad M, El Nozahi M. Comparison of the Efficacy and Safety of Sacubitril/ Valsartan versus Ramipril in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2021;143:7-13. doi:10.1016/j.amjcard.2020.12.037.
388. Shah AM, Claggett B, Prasad N, et al. Impact of Sacubitril/Valsartan Compared With Ramipril on Cardiac Structure and Function After Acute Myocardial Infarction: The PARADISE-MI Echocardiographic Substudy. Circulation. 2022;146:1067-81. doi:10.1161/ CIRCULATIONAHA.122.059210.
389. Xiong B, Nie D, Qian J, et al. The benefits of sacubitril-valsartan in patients with acute myocardial infarction: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(6): 4852-62. doi:10.1002/ehf2.13677.
390. Yang P, Han Y, Lian C, Wu X. Efficacy and safety of sacubitril/valsartan vs. valsartan in patients with acute myocardial infarction: A meta-analysis. Front Cardiovasc Med. 2022;9:988117. doi:10.3389/fcvm.2022.988117.
391. Zhou X, Zhu H, Zheng Y, et al. A systematic review and meta-analysis of sacubitrilvalsartan in the treatment of ventricular remodeling in patients with heart failure after acute myocardial infarction. Front Cardiovasc Med. 2022;9:953948. doi:10.3389/fcvm. 2022.953948.
392. McMurray JJ, Packer M, Desai AS, et al.; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi:10.1056/NEJMoa1409077.
393. Prabhakar M, Massel D. Adjunctive treatment with eplerenone reduced morbidity and mortality in acute myocardial infarction. ACP J Club. 2003;139(2):32.
394. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
395. Bossard M, Binbraik Y, Beygui F, et al. Mineralocorticoid Receptor Antagonists in Patients with Acute Myocardial Infarction — A Systematic Review and Meta-analysis of Randomized Trials. Am Heart J. 2018;195:60-9.
396. Schwartz GG, Olsson AG, Ezekowitz MD, et al.; for the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of Atorvastatin on Early Recurrent Ischemic Events in Acute Coronary Syndromes. The MIRACL Study: A Randomized Controlled Trial. JAMA. 2001;285:1711-8.
397. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-504.
398. Hulten E, Jackson JL, Douglas K, et al. The Effect of Early, Intensive Statin Therapy on Acute Coronary Syndrome. A Meta-analysis of Randomized Controlled Trials. Arch Intern Med. 2006;166:1814-21.
399. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-81.
400. Cholesterol Treatment Trialists Collaboration, Fulcher J, O'Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385: 1397-405.
401. Navarese EP, Kowalewski M, Andreotti F, et al. Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2014;113:1753-64. doi:10.1016/j.amjcard.2014.02.034.
402. Nusca A, Melfi R, Patti G, Sciascio GD. Statin loading before percutaneous coronary intervention: proposed mechanisms and applications. Future Cardiol. 2010;6(5):579-89. doi:10.2217/fca.10.77.
403. Cannon CP, Blazing MA, Giugliano RP, et al.; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
404. Jukema JW, Szarek M, Zijlstra LE, et al. ODYSSEY OUTCOMES Committees and Investigators. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome: ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;74(9):1167-76. doi:10.1016/j.jacc.2019.03.013.
405. Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74(20):2452-62. doi:10.1016/j.jacc.2019.08.010.
406. Trankle CR, Wohlford G, Buckley LF, et al. Alirocumab in acute myocardial infarction: results from the Virginia commonwealth university alirocumab response trial (VCU-AlirocRT). J Cardiovasc Pharmacol. 2019;74(3):266-9. doi:10.1097/FJC.0000000000000706.
407. Räber L, Ueki Y, Otsuka T, et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA. 2022;327(18):1771-81. doi:10.1001/jama. 2022.5218.
408. Ray KK, Wright RS, Khaled D, et al.; ORION-10 and ORION-11 Investigators. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 2020;382(16):1507-19. doi:10.1056/NEJMoa1912387.
409. Lee J, Egolum U, Parihar H, et al. Effect of Ezetimibe Added to High-Intensity Statin Therapy on Low-Density Lipoprotein Cholesterol Levels: A Meta-Analysis. Cardiol Res. 2021;12(2):98-108. doi:10.14740/cr1224.
410. Wang X, Wen D, Chen Y, et al. PCSK9 inhibitors for secondary prevention in patients with cardiovascular diseases: a bayesian network meta-analysis. Cardiovasc Diabetol. 2022;21(1):107. doi:10.1186/s12933-022-01542-4.
411. Ray KK, Reeskamp LF, Laufs U, et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J. 2022;43(8):830-3. doi:10.1093/eurheartj/ehab718.
412. Burnett H, Fahrbach K, Cichewicz A, et al. Comparative efficacy of non-statin lipidlowering therapies in patients with hypercholesterolemia at increased cardiovascular risk: a network meta-analysis. Curr Med Res Opin. 2022;38(5):777-84. doi:10.1080/03007995.2022.2049164.
413. Khan SA, Naz A, Qamar Masood M, Shah R. Meta-Analysis of Inclisiran for the Treatment of Hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi:10.1016/j.amjcard.2020.08.018.
414. Ray KK, Raal FJ, Kallend DG, et al.; ORION Phase III investigators. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44(2):129-38. doi:10.1093/eurheartj/ehac594.
415. Sabatine MS, Giugliano RP, Keech AC, et al.; for the FOURIER Steering Committee and Investigators. Evolocumab and Clinical Outcomesin Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713-22.
416. Schwartz GG, Steg PG, Szarek M, et al.; for the ODYSSEY OUTCOMES Committees and Investigators. Аlirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097-107.
417. Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: An Analysis from FOURIER. Circulation. 2018;138:756-66.
418. NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-97. doi:10.1056/NEJMoa0810625.
419. Kovalaske MA, Gandhi GY. ACP Journal Club. Intensive glucose control increased risk for death and severe hypoglycemia in critically ill adults. Ann Intern Med. 2009;151(4):JC2-5. doi:10.7326/0003-4819-151-4-200908180-02005.
420. Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995; 26:57-65.
421. Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512-5.
422. Mamas MA, Neyses L, Fath-Ordoubadi F. A meta-analysis of glucose-insulin-potassium therapy for treatment of acute myocardial infarction. Exp Clin Cardiol. 2010; 15:e20-e24.
423. Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113: 2906-13.
424. Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302-8.
425. The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-56. doi:10.1056/NEJMoa012689.
426. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-63. doi:10.1056/NEJMoa003289.
427. Belliard G, Catez E, Charron C, et al. Efficacy of therapeutic hypothermia after outof-hospital cardiac arrest due to ventricular fibrillation. Resuscitation. 2007;75:252-9. doi:10.1016/j.resuscitation.2007.04.014.
428. Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N Engl J Med. 2013;369:2197-206. doi:10.1056/NEJMoa1310519.
429. Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med. 2021;384(24):2283-94. doi:10.1056/NEJMoa2100591.
430. Wolfrum S, Roedl K, Hanebutte A, et al. Temperature control after in-hospital cardiac arrest: a randomized clinical trial. Circulation. 2022;146:1357-66. doi:10.1161/circulationaha.122.060106.
431. Vaahersalo J, Hiltunen P, Tiainen M, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in Finnish intensive care units: the FINNRESUSCI study. Intensive Care Med. 2013;39:826-37. doi:10.1007/s00134-013-2868-1.
432. Okazaki T, Hifumi T, Kawakita K, Kuroda Y; Japanese Association for Acute Medicine outof-hospital cardiac arrest (JAAM-OHCA) registry. Targeted temperature management guided by the severity of hyperlactatemia for out-of-hospital cardiac arrest patients: a post hoc analysis of a nationwide, multicenter prospective registry. Ann Intensive Care. 2019;9(1):127. doi:10.1186/s13613-019-0603-y.
433. Callaway CW, Coppler PJ, Faro J, et al. Association of Initial Illness Severity and Outcomes After Cardiac Arrest With Targeted Temperature Management at 36 °C or 33 °C. JAMA Netw Open. 2020;3(7):e208215. doi:10.1001/jamanetworkopen.2020.8215.
434. Nishikimi M, Ogura T, Nishida K, et al. Outcome Related to Level of Targeted Temperature Management in Postcardiac Arrest Syndrome of Low, Moderate, and High Severities: A Nationwide Multicenter Prospective Registry. Crit Care Med. 2021;49(8):e741-e750. doi:10.1097/CCM.0000000000005025.
435. Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N Engl J Med. 2019;381(24):2327-37. doi:10.1056/NEJMoa190666.
436. Perkins GD, Graesner JT, Semeraro F, et al. European Resuscitation Council Guidelines 2021: executive summary. Resuscitation. 2021;161:1-60. doi:10.1016/j.resuscitation.2021.02.003.
437. Bonnefoy-Cudraz E, Bueno H, Casella G, et al. Editor's Choice – acute cardiovascular care association position paper on intensive cardiovascular care units: an update on their definition, structure, organisation and function. Eur Heart J Acute Cardiovasc Care. 2018;7:80-95. doi:10.1177/2048872617724269.
438. Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. Am. J. Cardiol. 1967;20(4):457-64.
439. Khot UN, Jia G, Moliterno DJ, et al. Prognostic importance of physical examination for heart failure in non-ST-elevation acute coronary syndromes: the enduring value of Killip classification. JAMA. 2003;290(16):2174-81.
440. Masip J, Peacock WF, Price S, et al., Acute Heart Failure Study Group of the Acute Cardiovascular Care Association and the Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur Heart J. 2018;39:1725.
441. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: non-invasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600.
442. ФGray A, Goodacre S, Newby DE, et al. 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142-51.
443. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2021;42:3599-726.
444. Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion–a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137-55. doi:10.1002/ejhf.1369.
445. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531-40.
446. Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389-93.
447. Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007;50:144-52.
448. Kozhuharov N, Goudev A, Flores D, et al. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. 2019;322: 2292-302.
449. Freund Y, Cachanado M, Delannoy Q, et al. Effect of an emergency department care bundle on 30-day hospital discharge and survival among elderly patients with acute heart failure: the ELISABETH randomized clinical trial. JAMA. 2020;324:1948-56.
450. Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205-9.
451. Gil V, Dominguez-Rodriguez A, Masip J, et al. Morphine use in the treatment of acute cardiogenic pulmonary edema and its effects on patient outcome: a systematic review. Curr Heart Fail Rep. 2019;16:81-8.
452. Miro O, Gil V, Martin-Sanchez FJ, et al.; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. Chest. 2017;152:821-32.
453. Caspi O, Naami R, Halfin E, Aronson D. Adverse dose-dependent effects of morphine therapy in acute heart failure. Int J Cardiol. 2019;293:131-6.
454. Hochman JS, Buller CE, Sleeper LA, et al.; for the SHOCK Investigators. Cardiogenic Shock Complicating Acute Myocardial Infarction — Etiologies, Management and Outcome: A Report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1063-70.
455. Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544-58.
456. Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1315-41.
457. Maack C, Eschenhagen T, Hamdani N, et al. Treatments targeting inotropy. Eur Heart J. 2019;40:3626-44.
458. Mebazaa A, Motiejunaite J, Gayat E, et al.; ESC Heart Failure Long-Term Registry Investigators. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology heart failure long-term registry. Eur J Heart Fail. 2018;20:332-41.
459. De Backer D, Biston P, Devriendt J, et al.; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-89.
460. Levy B, Clere-Jehl R, Legras A, et al. Collaborators. Epinephrine versus norepine-phrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018; 72:173-82.
461. Leopold V, Gayat E, Pirracchio R, et al. Epinephrine and short-term survival in cardiogenic shock: an individual data meta-analysis of 2583 patients. Intensive Care Med. 2018;44:847-56.
462. Hajjar LA, Teboul J. Mechanical Circulatory Support Devices for Cardiogenic Shock: State of the Art. Crit Care. 2019;23:76. doi:10.1186/s13054-019-2368-y.
463. Thiele H, Zeymer U, Neumann FJ, et al.; Investigators I-SIT. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287-96.
464. Thiele H, Zeymer U, Neumann FJ, et al., Intraaortic Balloon Pump in cardiogenic shock IIti. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-45.
465. Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;3:CD007398.
466. Thiele H, Zeymer U, Thelemann N, et al.; Investigators IIT. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation. 2019;139(3):395-403. doi:10.1161/CIRCULATIONAHA.118.038201.
467. Dumas F, Bougouin W, Geri G, et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: insights from the PROCAT II registry. JACC Cardiovasc Interv. 2016;9:1011-8.
468. Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3:200-7. doi:10.1161/circinterventions.109.913665.
469. Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629-33. doi:10.1056/ nejm199706053362302.
470. Nademanee K, Taylor R, Bailey WE, et al. Treating electrical storm: sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation. 2000;102:742-7. doi:10.1161/01.cir.102.7.742.
471. Miwa Y, Ikeda T, Mera H, et al. Effects of landiolol, an ultra-short-acting beta1-selective blocker, on electrical storm refractory to class III antiarrhythmic drugs. Circ J. 2010;74:856-63. doi:10.1253/circj.cj09-0772.
472. Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-ofhospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341:871-8. doi:10.1056/nejm199909163411203.
473. Levine JH, Massumi A, Scheinman MM, et al. Intravenous amiodarone for recurrent sustained hypotensive ventricular tachyarrhythmias. J Am Coll Cardiol. 1996;27:67-75. doi:10.1016/0735-1097(95)00427-0.
474. Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-83.
475. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or animplantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-37.
476. Deedwania PC, Singh BN, Ellenbogen K, et al. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs Congestive Heart failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT). Circulation. 1998;98:2574-9. doi:10.1161/01.cir.98.23.2574.
477. Hofmann R, Steinwender C, Kammler J, et al. Intravenous amiodarone bolus for treatment of atrial fibrillation in patients with advanced congestive heart failure or cardiogenic shock. Wien Klin Wochenschr. 2004;116:744-9. doi:10.1007/s00508-004-0264-0.
478. Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone: a randomized, digoxincontrolled study. Eur Heart J. 1995;16:521-8. doi:10.1093/oxfordjournals.eurheartj.a060945.
479. Segal JB, McNamara RL, Miller MR, et al. The evidence regarding the drugs used for ventricular rate control. J Fam Pract. 2000;49:47-59.
480. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038-45. doi:10.1093/eurheartj/ehn579.
481. Batra G, Svennblad B, Held C, et al. All types of atrial fibrillation in the setting of myocardial infarction are associated with impaired outcome. Heart. 2016;102:926-33. doi:10.1136/ heartjnl-2015-308678.
482. Siu CW, Jim MH, Ho HH, et al. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007;132:44-9. doi:10.1378/chest.06-2733.
483. Feigl D, Ashkenazy J, Kishon Y. Early and late atrioventricular block in acute inferior myocardial infarction. J Am Coll Cardiol. 1984;4:35-8. doi:10.1016/s0735-1097(84)80315-0.
484. Brady WJ, Swart G, DeBehnke DJ, et al. The efficacy of atropine in the treatment of hemodynamically unstable bradycardia and atrioventricular block: prehospital and emergency department considerations. Resuscitation. 1999;41:47-55. doi:10.1016/s0300-9572(99)00032-5.
485. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A. Frequency and significance of highdegree atrioventricular block and sinoatrial node dysfunction in patients with non-STelevation myocardial infarction. Am J Cardiol. 2018;122(10):1598-603.
486. Singh SM, FitzGerald G, Yan AT, et al. High-grade atrioventricular block in acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. European Heart Journal. 2015;36:976-83. doi:10.1093/eurheartj/ehu357.
487. Damluji AA, van Diepen S, Katz JN, et al. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation. 2021;144:e16-e35. doi:10.1161/cir.0000000000000985.
488. Matteucci M, Fina D, Jiritano F, et al. The use of extracorporeal membrane oxygenation in the setting of postinfarction mechanical complications: outcome analysis of the Extracorporeal Life Support Organization Registry. Interact Cardiovasc Thorac Surg. 2020;31:369-74. doi:10.1093/icvts/ivaa108.
489. Alerhand S, Adrian RJ, Long B, Avila J. Pericardial tamponade: A comprehensive emergency medicine and echocardiography review. Am J Emerg Med. 2022;58:159-74. doi:10.1016/j.ajem.2022.05.001.
490. Gong FF, Vaitenas I, Malaisrie SC, Maganti K. Mechanical complications of acute myocardial infarction: a review. JAMA Cardiol. 2021;6:341-9. doi:10.1001/jamacardio.2020. 3690.
491. Ronco D, Matteucci M, Ravaux JM, et al. Mechanical circulatory support as a bridge to definitive treatment in post-infarction ventricular septal rupture. JACC Cardiovasc Interv. 2021;14:1053-66. doi:10.1016/j.jcin.2021.02.046.
492. Kilic A, Sultan I, Chu D, et al. Mitral valve surgery for papillary muscle rupture: outcomes in 1342 patients from the society of thoracic surgeons database. Ann Thorac Surg. 2020;110:1975-81. doi:10.1016/j.athoracsur.2020.03.
493. Valle JA, Miyasaka RL, Carroll JD. Acute mitral regurgitation secondary to papillary muscle tear: is transcatheter edge-to-edge mitral valve repair a new paradigm? Circ Cardiovasc Interv. 2017;10:e005050. doi:10.1161/circinterventions.117.005050.
494. Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36: 2921-64. doi:10.1093/eurheartj/ehv318.
495. Verma BR, Montane B, Chetrit M, et al. Pericarditis and post-cardiac injury syndrome as a sequelae of acute myocardial infarction. Curr Cardiol Rep. 2020;22:127. doi:10.1007/s11886-020-01371-5.
496. Weinsaft JW, Kim J, Medicherla CB, et al. Echocardiographic algorithm for postmyocardial infarction LV thrombus: a gatekeeper for thrombus evaluation by delayed enhancement CMR. JACC Cardiovasc Imaging. 2016;9:505-15. doi:10.1016/j.jcmg.2015.06.017.
497. Dalia T, Lahan S, Ranka S, et al. Warfarin versus direct oral anticoagulants for treating left ventricular thrombus: a systematic review and meta-analysis. Thromb J. 2021;19:7. doi:10.1186/s12959-021-00259-w.
498. Dou Q, Wang W, Wang H, et al. Prognostic value of frailty in elderly patients with acute coronary syndrome: a systematic review and meta-analysis. BMC Geriatr. 2019;19(1):222. doi:10.1186/s12877-019-1242-8.
499. Man C, Xiang S, Fan Y. Frailty for predicting all-cause mortality in elderly acute coronary syndrome patients: A meta-analysis. Ageing Res Rev. 2019;52:1-6. doi:10.1016/j.arr.2019.03.003.
500. Reiter M, Twerenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32:1379-89. doi:10.1093/eurheartj/ehr033.
501. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387:1057-65. doi:10.1016/s0140-6736(15)01166-6.
502. Bueno H, Betriu A, Heras M, et al. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. 2011;32:51-60. doi:10.1093/eurheartj/ehq375.
503. Bach RG, Cannon CP, Giugliano RP, et al. Effect of simvastatin-ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2019;4:846-54. doi:10.1001/jamacardio.2019.23.
504. Savonitto S, Ferri LA, Piatti L, et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. 2018;137:2435-45. doi:10.1161/circulationaha.117.032180.
505. Roe MT, Armstrong PW, Fox KA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297-309. doi:10.1056/NEJMoa1205512.
506. Xu W, Cai Y, Liu H, et al. Frailty as a predictor of all-cause mortality and readmission in older patients with acute coronary syndrome: A systematic review and meta-analysis. Wien Klin Wochenschr. 2020;132(11-12):301-9. doi:10.1007/s00508-020-01650-9.
507. Damluji AA, Forman DE, Wang TY, et al.; American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Lifestyle and Cardiometabolic Health. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147(3):e32-e62. doi:10.1161/CIR.0000000000001112.
508. Richter D, Guasti L, Walker D, et al. Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of CardioOncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol. 2022;29:216-27. doi:10.1093/eurjpc/zwaa167.
509. Batty J, Qiu W, Gu S, et al. One-year clinical outcomes in older patients with non-ST elevation acute coronary syndrome undergoing coronary angiography: an analysis of the ICON1 study. Int J Cardiol. 2019;274:45-51. doi:10.1016/j.ijcard.2018.09.086.
510. Gu SZ, Beska B, Chan D, et al. Cognitive decline in older patients with non-ST elevation acute coronary syndrome. J Am Heart Assoc. 2019;8:e011218. doi:10.1161/jaha.118.011218.
511. Ambrosetti M, Abreu A, Corrà U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2021;28(5):460-95. doi:10.1177/2047487320913379.
512. Salzwedel A, Jensen K, Rauch B, et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur J Prev Cardiol. 2020;27:1756-74. doi:10.1177/2047487320905719.
513. Santiago de Araújo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of cardiac rehabilitation dose on mortality and morbidity: a systematic review and meta-regression analysis. Mayo Clin Proc. 2017;92:1644-59. doi:10.1016/j.mayocp.2017.07.019.
514. van Halewijn G, Deckers J, Tay HY, et al. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: a systematic review and meta-analysis. Int J Cardiol. 2017;232:294-303. doi:10.1016/j.ijcard.2016.12.125.
515. Aronov DM, Krasnitsky VB, Bubnova MG, et al. The effect of physical training on physical performance, hemodynamics, blood lipids, clinical course and prognosis in patients with coronary artery disease after acute coronary events during complex rehabilitation and secondary prevention at the outpatient stage (Russian cooperative study). Kardiologiia. 2009;(3):49-56. (In Russ.) Аронов Д. М., Красницкий В. Б., Бубнова М. Г. и др. Влияние физических тренировок на физическую работоспособность, гемодинамику, липиды крови, клиническое течение и прогноз у больных ишемической болезнью сердца после острых коронарных событий при комплексной реабилитации и вторичной профилактике на амбулаторно-поликлиническом этапе (Российское кооперативное исследование). Кардиология. 2009;(3):49-56.
516. Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21:45-53. doi:10.1177/1357633x14562732.
517. Thomas RJ, Beatty AL, Beckle TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69-e89. doi:10.1161/CIR.0000000000000663.
518. Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:CD007130. doi:10.1002/14651858.CD007130.pub4.
519. Moulson N, Bewick D, Selway T, et al. Cardiac Rehabilitation During the COVID-19 Era: Guidance on Implementing Virtual Care. Can J Cardiol. 2020;36(8):1317-21. doi:10.1016/j.cjca.2020.06.006.
520. Abreu A, Frederix I, Dendale P, et al. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: the avenue towards EAPC accreditation programme: a position statement of the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2020;28:496-509. doi:10.1177/2047487320924912.
521. Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11:Cd001800. doi:10.1002/14651858.CD001800.pub4.
522. Ivanova GE, Melnikova EV, Shmonin AA, et al. Application of the international classification of functioning in the process of medical rehabilitation. Bulletin of Restorative Medicine. 2018;(6):2-77. (In Russ.) Иванова Г. Е., Мельникова Е. В., Шмонин А. А. и др.Применение международной классификации функционирования в процессе медицинской реабилитации. Вестник восстановительной медицины. 2018;(6):2-77.
523. Bubnova MG, Aronov DM. Cardiorehabilitation: stages, principles and international classification of functioning. Preventive medicine. 2020:23(5):40-9. (In Russ.) Бубнова М. Г., Аронов Д. М. Кардиореабилитация: этапы, принципы и международная классификация функционирования. Профилактическая медицина. 2020:23(5):40-9. doi:10.17116/profmed20202305140.
524. Guazzi M, Adams V, Conraads V, et al. European Association for Cardiovascular Prevention & Rehabilitation; American Heart Association. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261-74. doi:10.1161/CIR.0b013e31826fb946.
525. Fletcher GF, Ades PA, Kligfield P, et al.; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8): 873-934. doi:10.1161/CIR.0b013e31829b5b44.
526. Long L, Anderson L, He J, et al. Exercise-based cardiac rehabilitation for stable angina: Systematic review and meta-analysis. Open Heart. 2019;6:e000989.
527. Fidalgo ASF, Farinatti P, Borges JP, et al. Institutional Guidelines for Resistance Exercise Training in Cardiovascular Disease: A Systematic Review. Sports Med. 2019;49:463-75.
528. Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation postmyocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571-84.
529. Mezzani A, Hamm LF, Jones AM, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. European Journal of Preventive Cardiology. 2013;20:442-67.
530. Аронов Д. М., Бубнова М. Г., Барбараш О. Л. и др. Острый инфаркт миокарда с подъемом сегмента ST электрокардиограммы: реабилитация и вторичная профилактика. Российский кардиологический журнал. 2015;(1):6-52. doi:10.15829/1560-4071-2015-1-6-52.
531. Бубнова М. Г., Аронов Д. М., Красницкий В. Б. и др. Программа домашних физических тренировок после острого коронарного синдрома и/или эндоваскулярного вмешательства на коронарных артериях: эффективность и проблема мотивации больных. Терапевтический Архив. 2014;86(1):23-32.
532. Hansen D, Bonné K, Alders T, et al. Exercise training intensity determination in cardiovascular rehabilitation: Should the guidelines be reconsidered? Eur J Prev Cardiol. 2019;26(18):1921-8. doi:10.1177/2047487319859450.
533. Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16(Suppl 1):55-8.
534. Hannan AL, Hing W, Simas V, et al. High-intensity interval training versus moderateintensity continuous training within cardiac rehabilitation: a systematic review and metaanalysis. Open Access J Sports Med. 2018;9:1-17. doi:10.2147/OAJSM.S150596.
535. Conraads VM, Pattyn N, De Maeyer C, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203-10. doi:10.1016/j.ijcard.2014.10.155.
536. Pattyn N, Vanhees L, Cornelissen VA, et al. The long-term effects of a randomized trial comparing aerobic interval versus continuous training in coronary artery disease patients: 1-year data from the SAINTEX-CAD study. Eur J Prev Cardiol. 2016;23(11):1154-64. doi:10.1177/2047487316631200.
537. Sommaruga M, Angelino E, Della Porta P, et al. Best practice in psychological activities in cardiovascular prevention and rehabilitation: Position Paper. Monaldi Arch Chest Dis. 2018;88:966. doi:10.4081/monaldi.2018.966.
538. Pogosova N, Saner H, Pedersen SS, et al.; Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. Psychosocial aspects in cardiac rehabilitation: From theory to practice. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. Eur J Prev Cardiol. 2015;22(10):1290-306. doi:10.1177/2047487314543075.
539. Бойцов С. А., Погосова Н. В., Бадтиева В. А. и др. Кардиоваскулярная профилактика 2022. Российские национальные рекомендации. Российский кардиологический журнал. 2023;28(5):5452. doi:10.15829/1560-4071-2023-5452.
540. Aldcroft SA, Taylor NF, Blackstock FC, O'Halloran PD. Psychoeducational rehabilitation for health behavior change in coronary artery disease: a systematic review of controlled trials. J Cardiopulm Rehabil Prev. 2011;31:273-81.
541. Tully PJ, Ang SY, Lee EJ, et al. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2021; 12(12):CD008012. doi:10.1002/14651858.CD008012.pub4.
542. Richards SH, Anderson L, Jenkinson CE, et al. Psychological interventions for coronary heart disease: Cochrane systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:247-59.
543. Rutledge T, Redwine LS, Linke SE, Mills PJ. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med. 2013;75:335-49.
544. Ambrosetti M, Abreu A, Cornelissen V, et al. Delphi consensus recommendations on how to provide cardiovascular rehabilitation in the COVID-19 era. Eur J Prev Cardiol. 2021;28(5):541-57. doi:10.1093/eurjpc/zwaa080.
545. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-337. doi:10.1093/eurheartj/ehab484.
546. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86-97.
547. Thomson CC, Rigotti NA. Hospitaland clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45:459-79.
548. Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004; 328(7446):977-80. doi:10.1136/bmj.38055.715683.55.
549. Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031.
550. Chow CK, Jolly S, Rao-Melacini P, et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121:750-8.
551. Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837.
552. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103.
553. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329.
554. Mills EJ, Thorlund K, Eapen S, et al. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129:28-41.
555. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3: CD008286.
556. Lindson N, Chepkin SC, Ye W, et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2019, Issue 4. Art. No.: CD013308. doi:10.1002/14651858.CD013308.
557. Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5:CD000146. doi:10.1002/14651858.CD000146.pub5.
558. Howes S, Hartmann-Boyce J, Livingstone-Banks J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2020;4:CD000031. doi:10.1002/14651858. CD000031.pub5.
559. Cahill K, Lindson-Hawley N, Thomas KH, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;2016:CD006103. doi:10.1002/14651858.CD006103.pub7.
560. Trichopoulou A, Bamia C, Norat T, et al. Modified Mediterranean diet and survival after myocardial infarction: the EPIC-Elderly STudy. Eur J Epidemiol. 2007;22:871-81.
561. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189-96.
562. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonized meta-analysis. BMJ. 2019;366:l4570. doi:10.1136/ bmj.l4570.
563. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-29. doi:10.1007/s10654018-0380-1.
564. Freeman AM, Morris PB, Barnard N, et al. Trending Cardiovascular Nutrition Controversies. J Am Coll Cardiol. 2017;69:1172-87.
565. Miller V, Mente A, Dehghan M, et al. Prospective Urban Rural Epidemiology study i. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390:2037-49.
566. Wood AM, Kaptoge S, Butterworth AS, et al.; Emerging Risk Factors Collaboration E-CVDUKBASG. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599912 current drinkers in 83 prospective studies. Lancet. 2018;39:1513-23.
567. Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 19902016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015-35.
568. Pack QR, Rodriguez-Escudero JP, Thomas RJ, et al. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc. 2014;89:1368-77.
569. Jackson Ch L, Joshy G, Lewington S, et al. Body-mass index and all-cause mortality: individual participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-86.
570. Khan SS, Ning H, Wilkins JT, et al. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018;3:280-7.
571. Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399:1876-85. doi:10.1016/s01406736(22)00122-2.
572. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-85. doi:10.1161/01.cir.99.6.779.
573. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi:10.1056/NEJMoa1800389.
574. Connor J, Hall W. Thresholds for safer alcohol use might need lowering. Lancet. 2018;391(10129):1460-1. doi:10.1016/S0140-6736(18)30545-2.
575. Holmes MV, Dale CE, Zuccolo L, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi:10.1136/bmj.g4164.
576. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and metaregression analyses of randomized trials. J Hypertens. 2014;32:2285-95.
577. SPRINT Research Group, Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373: 2103-16.
578. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387: 957-67.
579. Pfeffer MA. ACE inhibitors in acute myocardial infarction: patient selection and timing. Circulation. 1998;97(22):2192-4. doi:10.1161/01.cir.97.22.2192.
580. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials. Diabetes Care. 2009;32:187-92.
581. American Diabetes Association. Glycemic Targets: Standards of Medical Care in Diabetes — 2019. Diabetes Care Jan. 2019;42 (Supplement 1):S61-S70.
582. American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes – 2019. Diabetes Care Jan. 2019;42 (Supplement 1): S90-S102.
583. UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control With Sulphonylureas or Insulin Compared With Conventional Treatment and Risk of Complications in Patients With Type 2 Diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-53.
584. Gurfinkel E, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) study. Eur Heart J. 2004;25:25-31.
585. Ciszewski A, Bilinska ZT, Brydak LB, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD Study. Eur Heart J. 2008;29:1350-8. doi:10.1093/eurheartj/ehm581.
586. Clar C, Oseni Z, Flowers N, et al. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015(5):CD005050.
587. Caldeira D, Costa J, Vaz-Carneiro A. [Analysis of the Cochrane Review: Influenza Vaccines for Preventing Cardiovascular Disease. Cochrane Database Syst Rev. 2015;5:CD005050]. Acta Med Port. 2015;28:424-6.
588. MacIntyre CR, Mahimbo A, Moa AM, Barnes M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart. 2016;102:1953-6.
589. Caldeira D, Ferreira JJ, Costa J. Influenza vaccination and prevention of cardiovascular disease mortality. Lancet. 2018;391:426-7.
590. Fröbert O, Götberg M, Erlinge D, et al. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation. 2021; 144:1476-84.
591. Yedlapati SH, Khan SU, Talluri S, et al. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2021;10:e019636. doi:10.1161/jaha.120.019636.
592. Fonseca HAR, Furtado RHM, Zimerman A, et al. Influenza vaccination strategy in acute coronary syndromes: the VIP-ACS trial. Eur Heart J. 2022;43(41):4378-88. doi:10.1093/ eurheartj/ehac472.
593. Tardif JC, Kouz S, Waters DD, et al. Efficacy And safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497-505. doi:10.1056/NEJMoa1912388.
594. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838-47. doi:10.1056/NEJMoa2021372.
595. Thom S, Poulter N, Field J, et al.; UMPIRE Collaborative Group. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918-29.
596. Castellano JM, Sanz G, Penalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64:2071-82.
597. Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. 2022;387:967-77. doi:10.1056/NEJMoa2208275.
598. Astin F, Stephenson J, Probyn J, et al. Cardiologists' and patients' views about the informed consent process and their understanding of the anticipated treatment benefits of coronary angioplasty: a survey study. Eur J Cardiovasc Nurs. 2020;19:260-8. doi:10.1177/1474515119879050.
599. Scott JT, Thompson DR. Assessing the information needs of post-myocardial infarction patients: a systematic review. Patient Educ Couns. 2003;50:167-77. doi:10.1016/s07383991(02)00126-x.
600. Gherli R, Mariscalco G, Dalen M, et al. Safety of preoperative use of ticagrelor with or without aspirin compared with aspirin alone in patients with acute coronary syndromes undergoing coronary artery bypass grafting. JAMA Cardiol. 2016;1:921-8.
601. Nijjer SS, Watson G, Athanasiou T, Malik IS. Safety of clopidogrel being continued until the time of coronary artery bypass grafting in patients with acute coronary syndrome: a metaanalysis of 34 studies. Eur Heart J. 2011;32:2970-888.
602. Serebruany V, Bulaeva N, Golukhova E. Ticagrelor and heart surgery controversy: we may have better antiplatelet options. J Thorac Dis. 2016;8(11):3016-9. doi:10.21037/jtd.2016.11.26.
603. Qu J, Zhang D, Zhang H, et al. Preoperative clopidogrel and outcomes in patients with acute coronary syndrome undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2022;163(3):1044-52.e15. doi:10.1016/j.jtcvs.2020.03.118.
604. Slater DK, Hlatky MA, Mark DB, et al. Outcome in suspected acute myocardial infarction with normal or minimally abnormal admission electrocardiographic findings. Am Journal Cardiol. 1987;60:766-70.
605. Lev EI, Battler A, Behar S, et al. Frequency, characteristics, and outcome of patients hospitalized with acute coronary syndromes with undetermined electrocardiographic patterns. American Journal Cardiol. 2003;91:224-7.
606. Gallo R, Steinhubl SR, White HD, et al.; STEEPLE Investigators. Impact of anticoagulation regimens on sheath management and bleeding in patients undergoing elective percutaneous coronary intervention in the STEEPLE trial. Catheter Cardiovasc Interv. 2009;73(3):319-25. doi:10.1002/ccd.21764.
607. Montalescot G, White HD, Gallo R, et al.; STEEPLE Investigators. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med. 2006;355(10):1006-17. doi:10.1056/NEJMoa052711.
608. Xu Y, Qiu Z, Yang R, et al. Efficacy of mineralocorticoid receptor antagonists in postmyocardial infarction patients with or without left ventricular dysfunction. A metaanalysis of randomized controlled trials. Medicine. 2018;97:51(e13690). doi:10.1097/MD.0000000000013690.
609. Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. 2022;79(9):933-46. doi:10.1016/j.jacc.2022.01.018.
610. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-47. doi:10.1161/CIRCULATIONAHA.110.009449.
Об авторах
О. B. АверковРоссия
Москва
Г. К. Арутюнян
Москва
Д. В. Дупляков
Самара
Е. В. Константинова
Москва
Н. Н. Никулина
Рязань
Р. М. Шахнович
Москва
И. С. Явелов
Москва
А. Н. Яковлев
Санкт-Петербург
С. А. Абугов
Москва
Б. Г. Алекян
Москва
Д. М. Аронов
Москва
М. В. Архипов
Екатеринбург
О. Л. Барбараш
Кемерово
С. А. Бойцов
Москва
М. Г. Бубнова
Москва
Т. В. Вавилова
Санкт-Петербург
У. Ю. Васильева
Москва
А. С. Галявич
Казань
В. И. Ганюков
Кемерово
С. Р. Гиляревский
Москва
Е. П. Голубев
Москва
Е. З. Голухова
Москва
Д. А. Затейщиков
Москва
Ю. А. Карпов
Москва
Е. Д. Космачева
Краснодар
Ю. М. Лопатин
Волгоград
В. А. Марков
Томск
Е. В. Меркулов
Москва
Н. А. Новикова
Москва
Е. П. Панченко
Москва
Д. В. Певзнер
Москва
Н. В. Погосова
Москва
Д. М. Прасол
Санкт-Петербург
А. В. Протопопов
Красноярск)
Д. В. Скрыпник
Москва
Р. С. Тарасов
Москва
С. Н. Терещенко
Москва
С. А. Устюгов
Конфликт интересов:
Красноярск
А. В. Хрипун
Ростов-на-Дону
Е. А. Цебровская
Санкт-Петербург
С. В. Шалаев
(Тюмень
Е. В. Шляхто
Санкт-Петербург
А. В. Шпектор
(Москва
С. С. Якушин
Рязань
Рецензия
Для цитирования:
Аверков О.B., Арутюнян Г.К., Дупляков Д.В., Константинова Е.В., Никулина Н.Н., Шахнович Р.М., Явелов И.С., Яковлев А.Н., Абугов С.А., Алекян Б.Г., Аронов Д.М., Архипов М.В., Барбараш О.Л., Бойцов С.А., Бубнова М.Г., Вавилова Т.В., Васильева У.Ю., Галявич А.С., Ганюков В.И., Гиляревский С.Р., Голубев Е.П., Голухова Е.З., Затейщиков Д.А., Карпов Ю.А., Космачева Е.Д., Лопатин Ю.М., Марков В.А., Меркулов Е.В., Новикова Н.А., Панченко Е.П., Певзнер Д.В., Погосова Н.В., Прасол Д.М., Протопопов А.В., Скрыпник Д.В., Тарасов Р.С., Терещенко С.Н., Устюгов С.А., Хрипун А.В., Цебровская Е.А., Шалаев С.В., Шляхто Е.В., Шпектор А.В., Якушин С.С. Острый коронарный синдром без подъема сегмента ST электрокардиограммы. Клинические рекомендации 2024. Российский кардиологический журнал. 2025;30(5):6319. https://doi.org/10.15829/1560-4071-2025-6319. EDN: CXJUIB
For citation:
Averkov O.V., Harutyunyan G.K., Duplyakov D.V., Konstantinova E.V., Nikulina N.N., Shakhnovich R.M., Yavelov I.S., Yakovlev A.N., Abugov S.A., Alekyan B.G., Aronov D.M., Arkhipov M.V., Barbarash O.L., Boytsov S.A., Bubnova M.G., Vavilova T.V., Vasilyeva E.Yu., Galyavich A.S., Ganyukov V.I., Gilyarevsky S.R., Golubev E.P., Golukhova E.Z., Zateyshchikov D.A., Karpov Yu.A., Kosmacheva E.D., Lopatin Yu.M., Markov V.A., Merkulov E.V., Novikova N.A., Panchenko E.P., Pevzner D.V., Pogosova N.V., Prasol D.M., Protopopov A.V., Skrypnik D.V., Tarasov R.S., Tereshchenko S.N., Ustyugov S.A., Khripun A.V., Tsebrovskaya E.A., Shalaev S.V., Shlyakhto E.V., Shpektor A.V., Yakushin S.S. 2024 Clinical practice guidelines for Acute coronary syndrome without ST segment elevation electrocardiogram. Russian Journal of Cardiology. 2025;30(5):6319. (In Russ.) https://doi.org/10.15829/1560-4071-2025-6319. EDN: CXJUIB
JATS XML