Scroll to:
Optimizing implantation of cardiac resynchronization therapy: a randomized controlled trial of electrophysiological or anatomical left ventricular lead placement strategy
https://doi.org/10.15829/1560-4071-2025-5720
EDN: HLOYRZ
Abstract
There is a variety of cardiac resynchronization therapy (CRT) strategies and none has ultimate benefits over the others.
Aim. To evaluate the influence of two strategies of left ventricular (LV) electrode implantation on the development of cardiovascular events in patients with chronic cardiac failure.
Material and methods. This was a randomized controlled clinical trial designed to compare the effectiveness of traditional anatomy-guided LV lead positioning strategy towards the electrocardiography-guided implantation approach in an optimal branch of the coronary sinus vein, being the closest to the latest electrically activated myocardial region.
Results. We enrolled 63 patients with NYHA class III or IV chronic heart failure with ventricular dyssynchrony, an LV ejection fraction (LVEF) less than 35%, an LV end diastolic dimension exceeding 150 ml, a QRS interval over 130 ms. The survival time in electrocardiography-guided approach (study group) was equal to 11,22 months, which was significantly lower in the anatomy-guided approach (control group). Time to re-hospitalization in a study group was nearly two times longer as compared with that in patients from the control group (10,188 months versus 5,548 months). LVEF was significantly higher in the study group with median value equal to 39% versus that in the control group equal to 35% (р=0,002).
Conclusions. The results of the present study demonstrate that electrocardiographyguided approach has benefits over traditional anatomy-guided approach in terms of improved cardiac structure and function in patients with NYHA class III and IV heart failure associated with ventricular dyssynchrony.
For citations:
Sabitov E.T., Abdrakhmanov A.S., Orekhov A.Yu., Sabitova D.Zh. Optimizing implantation of cardiac resynchronization therapy: a randomized controlled trial of electrophysiological or anatomical left ventricular lead placement strategy. Russian Journal of Cardiology. 2025;30(2):5720. https://doi.org/10.15829/1560-4071-2025-5720. EDN: HLOYRZ
Introduction
A number of randomized and non-randomized clinical trials were conducted over past decades demonstrating many benefits of cardiac resynchronization therapy (CRT) in terms of quality of life improvement, advanced functional status and exercise capacity in patients with ventricular dyssynchrony [1]. A favorable effect of CRT has also been shown on disease progression, which manifests as left ventricular (LV) remodeling and a range of other outcome measures. Besides, CRT reduces mortality and improves overall patient survival [2]. With a little exception, most of these studies enrolled patients with New York Heart Association (NYHA) class III or IV heart failure, while very few of them enrolled NYHA class II patients [3].
Still, around one third of CRT patients fail to respond appropriately as they cannot reach certain treatment goals within the desired period of time [4]. Although much variability in defining non-response exists, these goals can be summarized as a failure to achieve clinical improvement (reduction on NYHA class, improved quality of life or exercise capacity, etc.) or a reduction in number of adverse events (hospitalization rates and/or death). Due to the natural course of disease, achieving non-progression could also be considered as one of the desired treatment outcomes in a certain category of patients [5].
There is a variety of CRT implantation strategies and none with an ultimate benefit over others. In anatomy-guided approach the device is implanted in lateral or posterolateral branch of the coronary sinus vein [6], while in echocardiography-guided approach the lead is implanted in the latest mechanically activated region [7]. In electrocardiography-guided approach the lead is positioned in the latest electrically activated zone [8]. Cardiovascular magnetic resonance-guided approach was proposed to deploy the LV lead away from myocardial scarring [9], and so did multimodality imaging-guided approach [10]. To clarify the clinical effectiveness of electrocardiography guided LV lead positioning strategy, we conducted a trial comparing it with traditional anatomy-guided approach towards the optimal branch of the coronary sinus vein, which is the closest to the latest electrically activated myocardial region.
Material and methods
Patients. We enrolled 63 patients which NYHA class III or IV chronic heart failure associated with ventricular dyssynchrony, an LV ejection fraction (LVEF) less than 35%, an LV end diastolic dimension exceeding 150 ml, a QRS interval over 130 ms.
Also, the enrolled patients received an optimal management of heart failure according to current guidelines for the past three months, and failed to respond appropriately. The exclusion criteria were: NYHA class II or I chronic heart failure and presence of contraindications to cardiac pacing. Before the study onset, local Ethics Committee approved the study protocol (protocol № 7, 30.05.2017), and all patients were informed about study goals, procedures, possible risks and benefits, and provided written informed consent. General characteristics of study population are presented in Table 1, while characteristics of medical therapy provided before intervention are presented in Table 2.
Study protocol. Before CRT implantation, all study participants were undergone to the following tests: evaluation of NYHA class; 6-minute walk test; glomerular filtration rate with Chronic Kidney Disease Epidemiology Collaboration equation; echocardiography with measurements of LVEF and of end-systolic and end-diastolic volumes; plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations; and QRS interval by a standard 12-lead electrocardiography. The studies were performed before device implantation and 12 months after. Patients were randomly assigned (1:1 scheme) to either electrocardiography guided LV lead positioning strategy (study group) or anatomy guided LV lead positioning strategy (control group).
Device implantation. The patients were implanted commercially available CRT systems with 3 pacing leads. Implantation protocol in the control group followed traditional anatomy-guided strategy [6]. In the study group, right ventricular (RV) pacing lead was commonly placed in the right ventricle apex. Coronary sinus was catheterized with subsequent phlebography and identification of branch optimal for implantation of the LV pacing lead. The intraventricular delay (RV-LV activation delay) was measured at each coronary sinus branch vein that was considered suitable and the LV lead was implanted to the branch with maximal electrical delay. The right atrial pacing lead was implanted to the right atrial appendage.
Follow-up and study endpoints. Study endpoints were defined: duration of QRS complex (primary endpoint), rehospitalization, and mortality (secondary endpoints). Rehospitalization was defined as admission to inpatient department with overnight stay due to heart failure with subsequent improvement following medical therapy. The cause of every lethal outcome was assessed by two different physicians blinded to the CRT implantation strategy. We categorized all sudden uncertain lethality as a sudden cardiac death. All data collected throughout the study period were entered into a specially designed database (Figure 1).
Statistical analysis. The Kolmogorov-Smirnov test was applied prior to other statistical tests to check for the normality of data distribution. As the data distribution has proven to be non- normal, continuous variables were expressed as a median (25th; 75th percentiles) and compared by Pearson's chi-square for qualitative variables and by or Mann-Whitney U-test for quantitative variables. The data were presented as mean (M), median (Me), standard error (SE), and 95% confidence interval (CI). Kaplan-Meier survival test was used to show 12-month survival and re-admission after CRT. We analyzed the endpoints based on the intention-to-treat principle, and a value of p<0,05 was considered to be statistically significant. All statistical tests were performed with the help of SPSS (Statistical Package for the Social Sciences) software, version 20.0 for Windows.
Results
Initially, the main clinical and laboratory characteristics of the patients did not change in the 2 groups, though it is worth noting a higher level of LV CSR in the study group and a slightly higher median of the 6-minute walk test (the difference was statistically insignificant), as well as a more frequent history of undergone myocardial infarction in the control group (Table 1, 2).
At the first stage of the analysis we evaluated the dynamics of the main parameters reflecting the degree of myocardial stress and severity of myocardial dyssynchrony before device implantation and 12 months after in both groups (Wilcoxon test); the results are presented in Table 3. As shown in the table, significant data were obtained in the dynamics of the primary end point (QRS dynamics) in both groups. However, when comparing the width of the QRS complex after 12 months, no significant differences were obtained in the study and control groups (136,66 sec. main, 137,81 sec. — control, p=0,51). Also, no significant dynamics of LVEF in the group of LV electrode implantation under ECG control was demonstrated (for the study and control group, p=0,699). At the same time, a more significant decrease in NT-proBNP concentration was obtained in the study group, amounting to 178,78 pg/ml after 12 months, and in the control group to 384,08 pg/ml (p=0,035).
Kaplan-Meier survival analysis at 12 postoperative months indicated that the survival time in study group was equal to 11,22 (95% CI: 10,614-11,823) months, which was significantly lower in the control group (Mean=7,29) (95% CI: 5,627-8,954).
According to Figure 2 A and 2 B, the survival curves showed significant difference between the study groups in survival and rehospitalization up to 12 months after CRT (log-rank test <0,001).
Time to re-hospitalization in patients from the study group was nearly two times longer as compared with that in patients from the control group (М=10,188 versus М=5,548) (Table 4).
Table 1
General characteristics of study population (n=63)
Age (Median (25;75))* | Groups | Test of difference | ||||||
Control group, n=31 | Study group, n=32 | χ2 | D.f. | p | ||||
N | % | N | % | |||||
Demographic data, smoking | ||||||||
Age (Median (25;75))* | 65 (56;70) | 63 (53;70) | 439,500 | -0,778 | 0,437 | |||
Gender | Female | 12 | 38,7 | 8 | 25,0 | 1,366 | 1 | 0,240 |
Male | 19 | 61,3 | 24 | 75,0 | ||||
Smoking | No | 24 | 77,4 | 27 | 84,4 | 0,494 | 1 | 0,482 |
Yes | 7 | 22,6 | 5 | 15,6 | ||||
Cardiac impairment/arrhythmias | ||||||||
NYHA class before intervention | III | 23 | 74,2 | 26 | 81,2 | 0,454 | 1 | 0,501 |
IV | 8 | 25,8 | 6 | 18,8 | ||||
Ischemic heart disease | No | 1 | 3,2 | 0 | 0,0 | 1,049 | 1 | 0,306 |
Yes | 30 | 96,8 | 32 | 100,0 | ||||
Postinfarction cardiosclerosis | No | 3 | 9,7 | 12 | 37,5 | 6,719 | 1 | 0,010 |
Yes | 28 | 90,3 | 20 | 6,5 | ||||
Ischemic cardiomyopathy | No | 5 | 16,1 | 3 | 9,4 | 0,648 | 1 | 0,421 |
Yes | 26 | 83,9 | 29 | 90,6 | ||||
Dilated cardiomyopathy | No | 31 | 100,0 | 32 | 100,0 | — | — | — |
Yes | 0 | 0,0 | 0 | 0,0 | ||||
Persistent atrial fibrillation | No | 22 | 71,0 | 25 | 78,1 | 0,426 | 1 | 0,514 |
Yes | 9 | 29,0 | 7 | 21,9 | ||||
Paroxysmal atrial fibrillation | No | 29 | 93,5 | 30 | 93,8 | 0,001 | 1 | 0,974 |
Yes | 2 | 6,5 | 2 | 6,2 | ||||
Presence of comorbidities | ||||||||
Stroke in past history | No | 28 | 90,3 | 29 | 90,6 | 0,002 | 1 | 0,967 |
Yes | 3 | 9,7 | 3 | 9,4 | ||||
Arterial hypertension | No | 3 | 9,7 | 2 | 6,2 | 0,253 | 1 | 0,615 |
Yes | 28 | 90,3 | 30 | 93,8 | ||||
Chronic kidney disease | No | 22 | 71,0 | 22 | 68,8 | 0,037 | 1 | 0,848 |
Yes | 9 | 29,0 | 10 | 31,2 | ||||
Type II diabetes mellitus | No | 21 | 70,0 | 30 | 93,8 | 5,984 | 1 | 0,014 |
Yes | 9 | 30,0 | 2 | 6,2 | ||||
Cardiac interventions in past history | ||||||||
Revascularization | No | 21 | 67,7 | 23 | 71,9 | 0,128 | 1 | 0,721 |
Yes | 10 | 32,3 | 9 | 28,1 | ||||
Heart surgery with the heart-lung machine | No | 29 | 93,5 | 32 | 100,0 | 2,132 | 1 | 0,144 |
Yes | 2 | 6,5 | 0 | 0,0 | ||||
Endovascular surgery | No | 25 | 80,6 | 24 | 75,0 | 0,290 | 1 | 0,590 |
Yes | 6 | 19,4 | 8 | 25,0 | ||||
Six minute walk test, meters (Median (25;75))* | 189 (150;220) | 211 (184;243) | 359,000 | -1,885 | 0,059 | |||
CKD-EPI (Median (25;75))*, ml/min/1,72 m2 | 60 (43;84) | 65 (46;85) | 434,500 | -0,641 | 0,521 | |||
NT-proBNP (Median (25;75))*, pg/ml | 458,90 (376,56;505,91) | 462,18 (411,04;501,59) | 476,000 | -0,275 | 0,783 | |||
QRS, ms | 158 (150;168) | 153 (150;178) | 458,000 | -0,523 | 0,601 | |||
Left ventricular end-diastolic volume, ml | 211 (161;256) | 217 (191;286) | 399,500 | -1,327 | 0,185 | |||
Left ventricular end-systolic volume, ml | 127 (103;173) | 159 (123;192) | 361,500 | -1,850 | 0,064 | |||
Left ventricular ejection fraction, % | 30 (25;33) | 26 (20;33) | 373,500 | -1,691 | 0,091 | |||
Note: * — quantitative data were compared using Mann-Whitney test.
Abbreviation: CKD-EPI — Chronic Kidney Disease Epidemiology Collaboration.
Table 2
Characteristics of medical therapy provided before intervention (n=63)
Variables | Groups | Test of difference | ||||||
Control group, n=31 | Study group, n=32 | χ2 | D.f. | p | ||||
N | % | N | % | |||||
Loop diuretics | No | 7 | 23,3 | 6 | 18,8 | 0,196 | 1 | 0,658 |
Yes | 23 | 76,7 | 26 | 81,2 | ||||
ACE-I/ARB | No | 12 | 40,0 | 14 | 46,7 | 0,271 | 1 | 0,602 |
Yes | 18 | 60,0 | 16 | 53,3 | ||||
Beta-blockers | No | 6 | 19,4 | 4 | 12,5 | 0,554 | 1 | 0,457 |
Yes | 25 | 80,6 | 28 | 87,5 | ||||
Mineralocorticoid Receptor Antagonists | No | 11 | 35,5 | 10 | 32,3 | 0,072 | 1 | 0,788 |
Yes | 20 | 64,5 | 21 | 67,7 | ||||
Digoxin | No | 14 | 45,2 | 18 | 56,2 | 0,775 | 1 | 0,379 |
Yes | 17 | 54,8 | 14 | 43,8 | ||||
Amiodarone | No | 21 | 70,0 | 19 | 59,4 | 0,764 | 1 | 0,382 |
Yes | 9 | 30,0 | 13 | 40,6 | ||||
Oral anticoagulants (warfarin, NOACs) | No | 27 | 90,0 | 28 | 90,3 | 0,002 | 1 | 0,966 |
Yes | 3 | 10,0 | 3 | 9,7 | ||||
Two-component antiplatelet therapy | No | 19 | 61,3 | 14 | 43,8 | 1,942 | 1 | 0,63 |
Yes | 12 | 38,7 | 18 | 56,2 | ||||
Abbreviations: ACE-I — angiotensin-converting-enzyme inhibitors, ARB — angiotensin II receptor blocker, NOACs — non‐vitamin K antagonist oral anticoagulants.
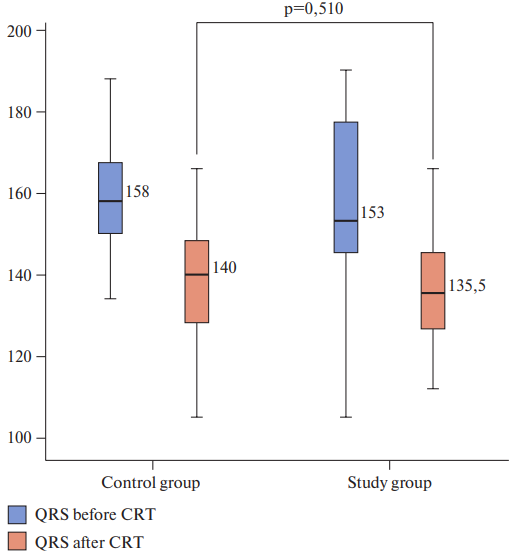
Figure 1. Duration of QRS complex (primary endpoint) before and after intervention, ms.
Table 4
Mean and median of survival time for the individual with "rehospitalization"
Group | Mean | Median | ||||||
Value, months | SE | Lower border | Upper border | Value, months | SE | Lower border | Upper border | |
Control group | 5,548 | 0,816 | 3,949 | 7,147 | 3,000 | 0,693 | 1,642 | 4,358 |
Study group | 10,188 | 0,547 | 9,115 | 11,260 | — | — | — | — |
Total | 7,905 | 0,569 | 6,789 | 9,020 | — | — | — | — |
Table 3
The main laboratory and instrumental performance indicators in the two groups
Parameter | Study group | р | Control group | р | ||
Before | After | Before | After | |||
Six-minute walk test, meters | 199,41±45,06 | 260,88±56,52 | <0,001 | 190,55±43,45 | 238,55±66,51 | <0,001 |
QRS, S | 156,0±21,75 | 137,66±15,07 | <0,001 | 159,16±15,52 | 137,81±12,86 | <0,001 |
NT-proBNP, pg/ml | 475,95 (422,38;510,62) | 178,79 (170,05;360,84) | <0,001 | 452,09 (384,46;477,66) | 384,08 (171,89;432,05) | <0,001 |
Left ventricular end-diastolic volume, ml | 210,25±74,90 | 180,38±73,36 | 0,002 | 242,00±72,55 | 217,61±55,56 | 0,006 |
Left ventricular end-systolic volume, ml | 134,50 (104,50-175,75) | 123,0 (101,75-151,75) | <0,001 | 158,0 (124,0-181,00) | 134,0 (106,00-163,50) | <0,001 |
Left ventricular ejection fraction, % | 25 (18;33) | 39 (29,0;40,0) | <0,001 | 30 (26,0;32,5) | 38,0 (34,5;40,0) | <0,001 |
Abbreviation: NT-proBNP — N-terminal pro-B-type natriuretic peptide.
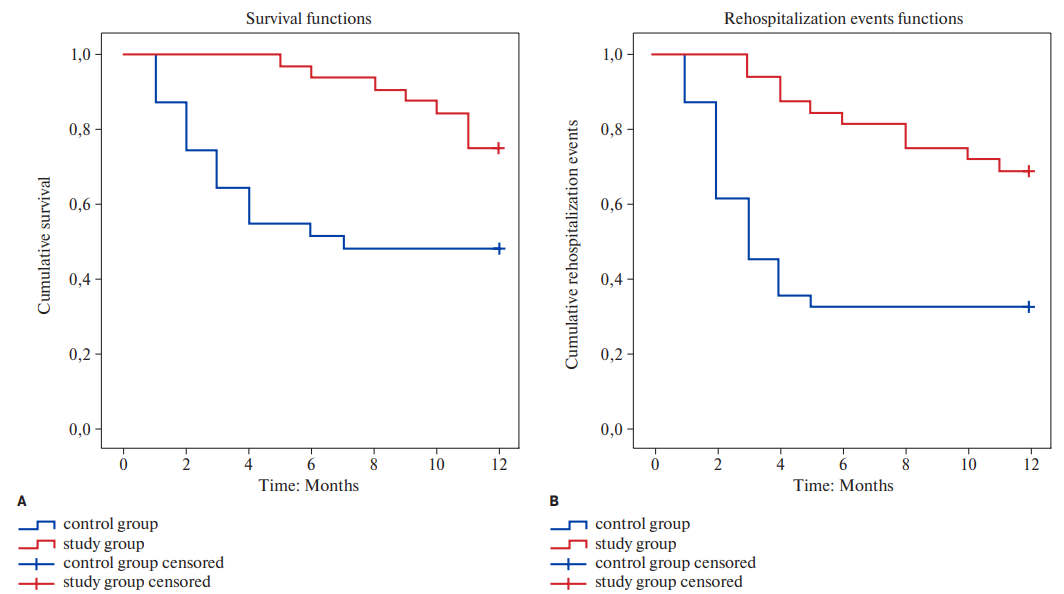
Figure 2. Kaplan-Meier curves examining survival (A) and rehospitalization (B) up to 12 months after CRT.
Discussion
The results of the present study demonstrate that LV lead positioning strategy based on RV-LV activation delay has benefits over traditional anatomy-guided approach in terms of improved cardiac structure and function in patients with NYHA class III and IV heart failure associated with ventricular dyssynchrony. These results are consistent with other studies demonstrating that heart failure patients may benefit from individualized LV lead positioning strategies to which belong both electrocardiography and echocardiography guided approaches [7][8]. Nevertheless, it has to be pointed-out that quit often the latest electrically and mechanically activated regions accord with lateral or posterolateral branch of the coronary sinus vein. In other words, the anatomy-guided approach fits the majority of patients with ventricular dyssynchrony [11].
Our study has certain similarities with earlier studies on electrocardiography guided approach. Such, in their retrospective study Fatemi and co-authors reported similar results on a group of patients with follow-up equal to 30±20 months. According to the authors, there was a correlation between lead positioning in the latest electrically activated zone and LV reverse remodeling [12]. The other prospective trial with shorter observation period (6 months) but larger study group (426 patients) found out that higher proportion of reverse remodeling was observed in patients underwent to electrocardiography guided approach [13]. Polasek and co-authors [14] conducted a retrospective study with one year follow-up on a cohort of 161 consecutive patients. The authors conclude that LV lead positioning strategy based on the latest electrically activated zone provides more favorable clinical response to CRT. Another larger prospective clinical trial with longer follow-up (2,2 years) demonstrated that longer RV-LV activation delay at the time of CRT implantation is associated with greater improvement in NT-proBNP levels, ejection fraction and showed better clinical outcomes [15].
Still, there are certain differences between our study and some of the abovementioned trials that have to be discussed. First, there was a substantial heterogeneity between the trials in terms of patient population. Three of the trials [12, 13, 15] enrolled patients with right bundle branch block, which was the exclusion criterion for Polasek and co-authors. Besides, Fatemi and co-authors excluded those patients having scar in the lateral wall. Also, these trials differed by the design (two trials had prospective nature and two were retrospective) and by the duration of follow-up. Second, only prospective trials [14][15] attempted to maximize the activation delay already at the time of implantation, which was similar with our strategy. In general, RV-LV delay appears to be more comprehensive approach since the location of RV lead also contributes to the clinical outcome of CRT. Third, different research groups applied dissimilar approaches for evaluation of successful resynchronization with CRT.
It appears to be rational to conclude that intraventricular delay may be a sign of significant electrical dyssynchrony, which could serve as an alternate marker for anticipating CRT benefits as compared with mechanical dyssynchrony. There is an opinion that left bundle branch block is an electrical disease for which CRT could be a potent therapy [16]. Thus, non-left bundle branch block patients may not be benefited from this strategy as the nature of their disease is more complex and needs further investigations [14].
One of the key findings of the present study was the significant increase in LVEF seen after 12 months in the study group. This may be attributed primarily to a decrease in end-systolic volume in patients whose LV lead positioning strategy was based on RV-LV activation delay as compared with that in patients from the control group. Such, our study group was characterized by the improved heart volumes, which allows us to suppose that CRT strategy based on RV-LV delay can play a positive role in the harmful pathophysiology of heart failure associated with ventricular dyssynchrony. Another key finding of the current study was a reduction of NT-proBNP levels, which is secreted by a heart wall in response to stress factors and serves for the prognosis of treatment outcomes [17]. As this reduction was more marked in the RV-LV delay group, we may conclude that these patients are better protected from negative postoperative cardiac events as compared with the patients from control group.
Our study has certain limitations. First, the sample size was relatively small (63 patients), which prevented us from conducting more detailed analyses. However, this was a prospective clinical trial with reasonable duration of follow-up (12 months). Still, many drug trials showed that a substantially longer follow-up is often required to demonstrate the full potential of antiarrhythmic agents [18]. Second, RV-LV activation delay may be influenced by baseline QRS duration and by coronary sinus branches optimal for implantation. Third, it is a common bias for all CRT studies to be limited by distribution of veins suitable for LV lead positioning and our study was not the exception.
Conclusion
Electrocardiography-based LV lead positioning approach assessed by RV-LV activation delay was found to be a significant predictor of QRS complex duration, mortality and rehospitalisation rates in CRT patients with NYHA class III and IV heart failure associated with ventricular dyssynchrony (QRC after CRT in control group was 135,5 ms, p=0,002). Therefore, this study has certain implications in terms of maximum efforts that should be made to optimize the LV lead positioning at the time of implantation.
Relationships and Activities: none.
References
1. Dauw J, Martens P, Mullens W. CRT Optimization: What Is New? What Is Necessary? Curr Treat Options Cardiovasc Med. 2019;21(9):45. doi:10.1007/s11936-019-0751-2.
2. Alraies MC, Buchanan K, Waksman R. CRT 2017 late-breaking trials. Cardiovasc Revasc Med. 2017;18(4):304-307. doi:10.1016/j.carrev.2017.04.014.
3. Santangeli P, Di Biase L, Pelargonio G et al. Cardiac resynchronization therapy in patients with mild heart failure: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2011;32(2):125-35. doi:10.1007/s10840-011-9584-y
4. Daubert C, Behar N, Martins RP et al. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. 2017;38(19):1463-1472. doi:10.1093/eurheartj/ehw270.
5. Mullens W, Verga T, Grimm RA et al. Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J Am Coll Cardiol. 2009; 53(7):600–7. doi:10.1016/j.jacc.2008.08.079.
6. Brignole M, Auricchio A, Baron-Esquivias G et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–329. doi:10.1093/europace/eut206.
7. Saba S, Marek J, Schwartzman D et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail. 2013;6(3):427–34. doi:10.1161/CIRCHEARTFAILURE.112.000078.
8. Singh JP, Berger RD, Doshi RN et al. Rationale and design for ENHANCE CRT: QLV implant strategy for non-left bundle branch block patients. ESC Heart Fail. 2018;5(6):1184–90. doi:10.1002/ehf2.12340
9. Leyva F, Foley PW, Chalil S et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13(1):29. doi:10.1186/1532-429X-13-29.
10. Sommer A, Kronborg MB, Norgaard BL et al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Eur J Heart Fail. 2016;18(11):1365-1374. doi:10.1002/ejhf.530
11. Lund LH, Svennblad B, Dahlstrom U et al. Effect of expanding evidence and evolving clinical guidelines on the prevalence of indication for cardiac resynchronization therapy in patients with heart failure. Eur J Heart Fail. 2018;20(4):769–7. doi:10.1002/ejhf.929
12. Fatemi M, Le Gal G, Blanc JJ et al. The use of epicardial electrogram as a simple guide to select the optimal site of left ventricular pacing in cardiac resynchronization therapy. Cardiol Res Pract. 2011:956062. doi:10.4061/2011/956062
13. Gold MR, Birgersdotter-Green U, Singh JP et al. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J. 2011;32(20):2516–2524. doi:10.1093/eurheartj/ehr329.
14. Polasek R, Kucera P, Nedbal P et al. Local electrogram delay recorded from left ventricular lead at implant predicts response to cardiac resynchronization therapy: retrospective study with 1 year follow up. BMC cardiovascular disorders. 2012;12:34. doi:10.1186/1471-2261-12-34
15. Kosztin A, Kutyifa V, Nagy VK et al. Longer right to left ventricular activation delay at cardiac resynchronization therapy implantation is associated with improved clinical outcome in left bundle branch block patients. Europace. 2016;18(4):550–559. doi:10.1093/europace/euv117.
16. Goldenberg I, Kutyifa V, Klein HU et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–701. doi:10.1056/NEJMoa1401426
17. Schouten O, Hoeks SE, Goei D et al. Plasma N-terminal pro-B-type natriuretic peptide as a predictor of perioperative and long-term outcome after vascular surgery. Journal of Vascular Surgery. 2009;49(2):435 – 442 doi:10.1016/j.jvs.2008.08.063.
18. Abraham WT, Young JB, León AR et al. Multicenter InSync ICD II Study Group. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110(18):2864-8. doi:10.1161/01.CIR.0000146336.92331.D1.
About the Authors
E. T. SabitovKazakhstan
PhD
Semey
Competing Interests:
none
A. S. Abdrakhmanov
Kazakhstan
Doctor of Medical Sciences, Associate Professor.
Astana
Competing Interests:
none
A. Yu. Orekhov
Kazakhstan
Semey
Competing Interests:
none
D. Zh. Sabitova
Kazakhstan
Master of Public Health.
Semey
Competing Interests:
none
Supplementary files
What is already known about the subject?
- Around one third of cardiac resynchronization therapy (CRT) patients fail to respond appropriately as they cannot reach certain treatment goals within the desired period of time. There is a variety of CRT implantation strategies and none has ultimate benefits over others.
What might this study add?
- Electrocardiography-based left ventricular lead positioning approach assessed by right ventricular-left ventricular activation delay was found to be a significant predictor of QRS complex duration, mortality and rehospitalisation rates in CRT patients with NIHA class III and IV heart failure associated with ventricular dyssynchrony.
Review
For citations:
Sabitov E.T., Abdrakhmanov A.S., Orekhov A.Yu., Sabitova D.Zh. Optimizing implantation of cardiac resynchronization therapy: a randomized controlled trial of electrophysiological or anatomical left ventricular lead placement strategy. Russian Journal of Cardiology. 2025;30(2):5720. https://doi.org/10.15829/1560-4071-2025-5720. EDN: HLOYRZ
JATS XML

















































