Scroll to:
Antiplatelet therapy de-escalation in a patient after percutaneous coronary intervention with a high risk of bleeding
https://doi.org/10.15829/1560-4071-2023-5274
Abstract
According to recommendations of ESC 2020-year, de-escalation of therapy with a P2Y12 receptor inhibitor (transition from prasugrel or ticagrelor to clopidogrel) It can be considered as an alternative strategy of dual antiplatelet therapy (DAPT) for patients with acute coronary syndrome (ACS) who are unsuitable for the use of a strong platelet inhibitor.
De-escalation can be performed based on an individual clinical evaluation under the supervision of platelet function testing or CYP2C19 genotyping, depending on the patient’s risk profile and the availability of appropriate diagnostic methods. The optimal dosage of strong P2Y12 receptor inhibitors, such as ticagrelor or prasugrel is not entirely clear and is especially difficult to define for patients of Asian nationality.
The article describes a clinical case of antiplatelet therapy de-escalation, particularly a dose reduction of the potent P2Y12 receptor inhibitor ticagrelor in a patient after percutaneous coronary intervention (PCI) with a high risk of bleeding based on platelet function determination and genetic testing. A 47-year-old patient of Kazakh nationality was hospitalized with gastrointestinal bleeding.
Given bleeding type 3 by BARC (Bleeding Academic Research Consortium) associated with DAPT, it was decided to apply a strategy to de-escalate antiplatelet therapy under the control of platelet function testing (PFT) and genetic testing.
In this case, replacement of ticagrelor with the weak P2Y12 receptor inhibitor clopidogrel was not possible as the patient appeared to be a carrier of the CYP2C19*2 polymorphism contributing to loss of function of the cytochrome P-450(CYP) enzyme.
Keywords
For citations:
Kassymova A.A., Mansurova J.A., Karazhanova L.K., Chinybayeva A.A. Antiplatelet therapy de-escalation in a patient after percutaneous coronary intervention with a high risk of bleeding. Russian Journal of Cardiology. 2023;28(5):5274. https://doi.org/10.15829/1560-4071-2023-5274
Fundamental aspects of coronary complication prevention in patients with after PCI in ACS include DAPT with aspirin and platelet P2Y12 receptor inhibitor [1]. With existing advanced generation of drug-eluting stents and increased use of potent P2Y12 receptor inhibitors, thrombotic events have dramatically decreased and the prevention of hemorrhagic complications has become the primary goal. The emphasis on reducing bleeding also arose due to an ever-increasing understanding of its prognostic consequences, including mortality [2]. This led to the concept of adapting DAPT by "de-escalating" P2Y12 receptor inhibitors as a bleeding reduction strategy in patients when the risk of thrombosis is reduced but the risk of bleeding persists [3][4].
Two different approaches are recommended in de-escalation strategies, according to a recent consensus expert report: with PFTs and on a genetic basis [5]. Especially may be justified for Asians having a different risk profile for both ischemia and bleeding compared with the Caucasoid population [6]. A different genetic profile as well as a higher prevalence of cytochrome P450 loss-of-function alleles CYP2C19*2 and *3 are associated with the highest incidence of high platelet reactivity [7].
However, the pharmacodynamic and pharmacokinetic response to potent P2Y12 receptor inhibitors differs in Asians from those of the Caucasian race. Exposure to the active metabolizer ticagrelor was higher in patients from China, Japan, and Korea (~30%) compared with Europeans even after adjustment for body weight [8]. Compared with clopidogrel, the standard dose of ticagrelor in the PHILO and TICAKOREA trials was associated with an increased risk of adverse outcomes, including massive bleeding, cardiovascular death, MI, or stroke [9].
To date, no comparative pharmacodynamic data on dose reduction strategies for potent P2Y12 inhibitors is available. In the HOPE-TAILOR trial in Korean ACS patients, a de-escalation strategy with half the dose of ticagrelor (45 mg) compared with the standard dose significantly increased the frequency of optimal platelet reactivity, and patients had no serious bleeding and ischemic complications were rare after 9 months of follow-up [10].
Clinical case
A 47-year-old patient of Kazakh nationality was hospitalized with gastrointestinal bleeding.
Complaints at admission: weakness, stabbing pains in the heart region, vomiting "coffee grounds", dark-colored stools (melena).
Deterioration during the day — repeated vomiting of coffee grounds, weakness, black stools.
From anamnesis: Two months ago, he had a myocardial infarction with ST-segment elevation of the lower wall of the left ventricle. An emergency PCI was performed with stenting of the right coronary arteries with two drug-eluting stents. On a DAPT scale of 3 points, long-term DAPT was shown. According to the European Association for Cardiothoracic Surgery (ESC) 2018 guidelines for myocardial revascularization, the patient received DAPT with ticagrelor 90 mg twice daily in combination with aspirin 100 mg and for gastroprotective therapy pantoprazole 40 mg 1 time a day [11]. A patient had gastrointestinal bleeding (GIB) 2 months after the start of DATT.
Arterial hypertension (AH) for 10 years. With a maximum lift up to 160/100 mm Hg. Heredity is burdened by hypertension. Bad habits: smokes for 10 years.
Laboratory sampling: troponin I — 0,1 ng/ml (up to 0,3 ng/ml); Complete blood count: HCT — 30%; leukocytes — 8,1/l; PLT — 162,0/l; erythrocytes — 2,8/l; HGB — 88 g/l.
Biochemistry: total protein — 66,6 g/l, urea — 4,9 mmol/l, creatinine — 93,0 µmol/l, glucose — 5,3 mmol/l, ALT — 0,06, AST — 0,10, total bilirubin — 15,06 mmol/l, direct bilirubin — 3,76, amylase — 16,0.
GFR based on Cockroft-Gault Formula equals to 94,1 ml/min/1,73 m2.
Coagulogram: INR — 1,1; PTI — 89%; PТ — 11,5; APTT — 25 sec; fibrinogen — 3,6 g/l.
Echocardiography data: the walls of the AO, the cusps of the AoC, MV are sealed. The cavities of the heart are not dilated. The contractility of the myocardium of the left ventricle is satisfactory (EF 52%). Hypokinesis of the inferior LV wall. Hypertrophy of IVS, PWLV. Diastolic dysfunction of LV.
On the electrocardiogram: Sinus rhythm with a heart rate of 88 bpm. Electrical axis of the heart (EAH) is deflected to the left. Cicatricial fields on the lower wall of the left ventricle. Signs of LV hypertrophy.
According to fibrogastroduodenoscopy: hernia of the esophageal opening of the diaphragm 1 st. Reflux esophagitis 1 st. Signs of mild atrophy of the gastric mucosa (C2). Gastric ulcer with bleeding, Forrest 1.
Clinical diagnosis: peptic ulcer of the stomach. Forrest 1.
Complication: posthemorrhagic anemia of moderate severity.
Accompanying: ischaemic heart disease. Single-vessel lesion of the coronary bed. Transferred myocardial infarction (myocardial infarction of the lower wall of the left ventricle). Chronic heart failure I, FC 2 according to NYHA with preserved ejection fraction. Arterial hypertension of the 2nd degree, risk 4.
The patient received conservative treatment for gastrointestinal bleeding. Hemostatic therapy stopped the bleeding.
Given bleeding 3 type from BARC (Bleeding Academic Research Consortium) associated with DAPT, it was decided to apply a strategy to de-escalate antiplatelet therapy under the control of PFTs and genetic testing.
Given the bleeding in the background of DAPT, platelet function was tested using the AggRAM Helena Biosciences Europe Aggregometer in response to stimulation with 10,0 μg/mL adenosine-5′-diphosphate (ADP).
The maximum percentage of residual platelet reactivity (RPR) was 6,1% and the area under the AUC curve was 0,16 (Fig. 1) corresponding to strong platelet inhibition by antiaggregant.
Pharmacogenetic testing revealed heterozygous carriage of the CYP2C19*2(G681A)-G/A allele and a normal genotype for the CYP2C19*3(Trp212Ter)-G/G allele (Fig. 2).
Given that the patient is a "slow clopidogrel metabolizer", replacement of ticagrelor with clopidogrel was not feasible. Therefore, ticagrelor was de-escalated from 90 mg 2 times a day to a reduced dose of 60 mg 2 times a day under ORT control. During bleeding, DAT was interrupted for three days. To prevent stent thrombosis, platelet aggregation analysis was performed. Taking into account positive dynamics, the absence of hemorrhagic phenomena, antiplatelet therapy was resumed on the fourth day with a reduced dose of ticagrelor 60 mg 2 times a day.
Thus, the reduced dose of the potent P2Y12 inhibitor ticagrelor 60 mg 2 times a day resulted in optimal suppression of platelet activity: the maximum percentage of platelet aggregation — 37,8% and the area under the AUC curve — 3,9 (Fig. 3).
The patient was discharged in a satisfactory condition and prescribed to take ticagrelor (60 mg twice a day) and for gastroprotective therapy pantoprazole (40 mg 1 time per day). As a result, there was no recurrence of bleeding and no ischemic events.
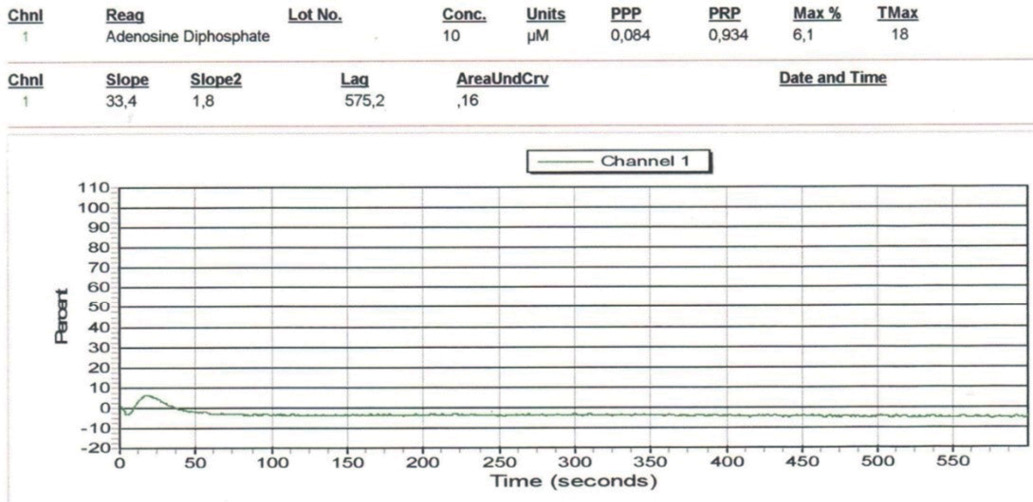
Fig. 1. Aggregatogram with 10,0 µg/ml ADP. Residual platelet reactivity against a standard dose of ticagrelor (the analysis was performed during bleeding).
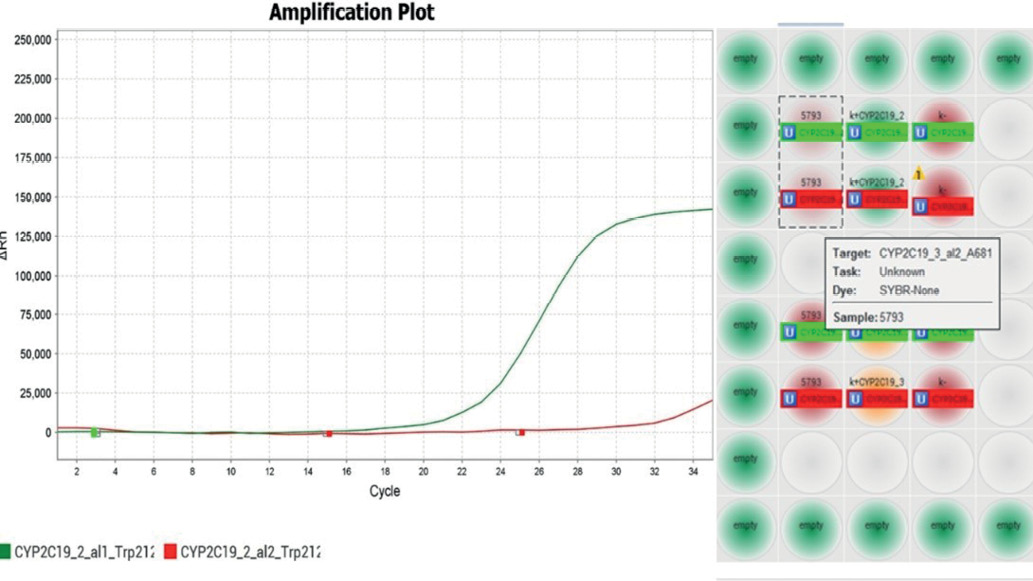
Fig. 2. The result of PCR analysis in the study of a DNA sample, heterozygous substitution in the CYP2C19*2(G681A)-G/A gene and the normal genotype for the CYP2C19*3(Trp212Ter)-G/G allele.
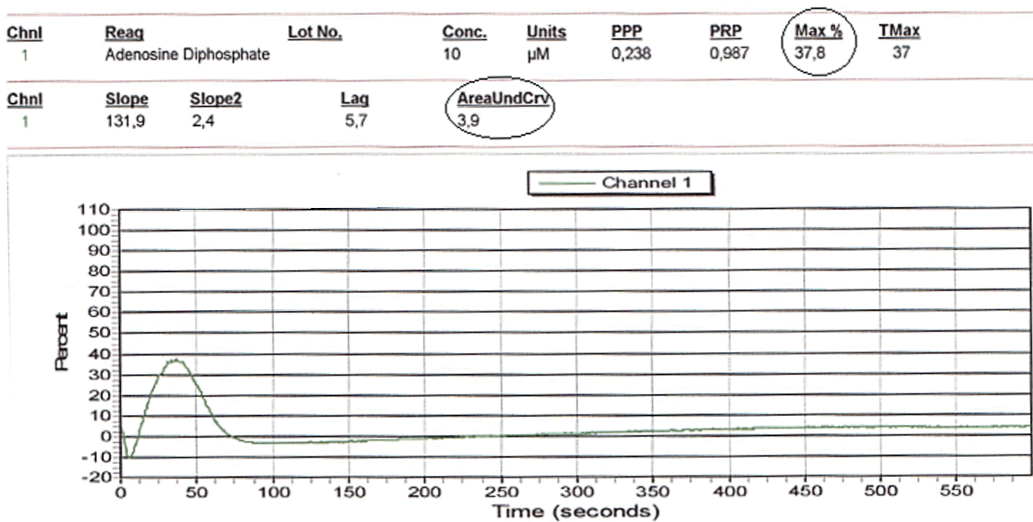
Fig. 3. Aggregatogram with 10,0 µg/ml ADP. Residual platelet reactivity on the background of reduced dose of ticagrelor (the analysis was performed on day 3 after ticagrelor dose reduction).
Discussion
According to the 2020 ESC Guidelines, DAPT of ASA and next-generation P2Y12 inhibitors prasugrel/ticagrelor are recommended after PCI in patients with ACS taking into account their higher efficacy [12].
When the risk of bleeding is high, de-escalation of P2Y12-inhibitory therapy (switching from the potent P2Y12 inhibitor prasugrel/ticagrelor to clopidogrel) under control of platelet function and genotyping for carrier CYP2C19 polymorphisms is recommended as an alternative DAPT strategy [5][12].
A lot of tests are used to analyze platelet function, but we still cannot control the effectiveness of antiplatelet therapy. The most difficult problem is the lack of correlation between the results of available tests due to the use of different protocols and aggregation inducers [13]. Optical aggregometry is still considered the "gold standard" for determining platelet function and remains the most common test, based on the turbidimetric method developed by G. Born in the 1960s [14]. In testing platelet function, an ADP inducer of 10,0 mcg/ml was used, since previously the parameters of platelet aggregation tests were standardized in the laboratory, and reference intervals with 5,0 mcg/ml, 10,0 mcg/ml of ADP were established [15]. In the studies, statistically significant results were obtained with the above concentration of ADP [16].
However, pharmacogenetic testing demonstrated that the patient had a heterozygous carrier of the CYP2C19*2(G681A)-G/A polymorphism associated with clopidogrel resistance. A number of studies evaluating the use of genetic testing for antiplatelet therapy decisions are underway and may add evidence for de-escalation based on platelet function [17].
Conclusion
In the clinical case we evaluated the pharmacodynamic effect and clinical outcome of a de-escalation strategy of reduced-dose ticagrelor 60 mg in a patient after PCI with a high risk of bleeding. De-escalation with a reduced dose of ticagrelor (60 mg) compared to the standard dose significantly increased the frequency of optimal platelet reactivity, the patient did not have repeat bleedings and no ischemic events were observed.
In order to ensure a balance between safety and efficacy in dual antiplatelet therapy de-escalation platelet function testing and genotyping for carriage of CYP2C19*2, *3 polymorphisms is required. Strategies for optimal dosage reduction of potent P2Y12 inhibitors require further study to balance safety and efficacy.
Relationships and Activities: none.
References
1. Capodanno D, Alfonso F, Levine GN, et al. ACC/AHA Versus ESC Guidelines on Dual Antiplatelet Therapy: JACC Guideline Comparison. J Am Coll Cardiol. 2018;72(23 Pt A):2915-31. doi:10.1016/j.jacc.2018.09.057.
2. Généreux P, Giustino G, Witzenbichler B, et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2015;66(9):1036-45. doi:10.1016/j.jacc.2015.06.1323.
3. Zettler ME, Peterson ED, McCoy LA, et al.; TRANSLATE-ACS Investigators. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: Insights from the Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) observational study. Am Heart J. 2017183:62-8. doi:10.1016/j.ahj.2016.10.006.
4. Sinnaeve PR, Van de Werf F. Clopidogrel instead of prasugrel or ticagrelor after 1 month in stabilized ACS patients: back to square one for DAPT? Eur Heart J. 2017;38(41):3079-81. doi:10.1093/eurheartj/ehx253.
5. Sibbing D, Aradi D, Alexopoulos D, et al. Updated Expert Consensus Statement on Pla¬telet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Per¬cuta¬neous Coronary Intervention. JACC Cardiovasc Interv. 2019;12(16):1521-37. doi:10.1016/j.jcin.2019.03.034.
6. Levine GN, Jeong YH, Goto S, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11(10):597-606. doi:10.1038/nrcardio.2014.104.
7. Jeong YH. "East asian paradox": challenge for the current antiplatelet strategy of "one-guideline-fits-all races" in acute coronary syndrome. Curr Cardiol Rep. 2014;16(5):485. doi:10.1007/s11886-014-0485-4.
8. Small DS, Kothare P, Yuen E, et al. The pharmacokinetics and pharmacodynamics of prasu¬grel in healthy Chinese, Japanese, and Korean subjects compared with healthy Caucasian subjects. Eur J Clin Pharmacol. 2010;66(2):127-35. doi:10.1007/s00228-009-0737-1.
9. Park DW, Kwon O, Jang JS, et al.; TICAKOREA Investigators. Clinically Significant Bleeding With Ticagrelor Versus Clopidogrel in Korean Patients With Acute Coronary Syndromes Intended for Invasive Management: A Randomized Clinical Trial. Circulation. 2019;140(23):1865-77. doi:10.1161/CIRCULATIONAHA.119.041766.
10. Jin CD, Kim MH, Song K, et al. Pharmacodynamics and Outcomes of a De-Escalation Strategy with Half-Dose Prasugrel or Ticagrelor in East Asians Patients with Acute Coronary Syndrome: Results from HOPE-TAILOR Trial. J Clin Med. 2021;10(12):2699. doi:10.3390/jcm10122699.
11. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi:10.1093/eurheartj/ehy394. Erratum in: Eur Heart J. 2019;40(37):3096.
12. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-367. doi:10.1093/eurheartj/ehaa575. Erratum in: Eur Heart J. 2021;42(19):1908. Erratum in: Eur Heart J. 2021;42(19):1925.
13. Mansurova JA, Karazhanova LK. Independent Predictors of Adverse Cardiovascular Events in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention During Hospitalization. Kardiologiia. 2018;58(12):22-9. (In Russ.) doi:10.18087/cardio.2018.12.10205.
14. Puchinian NF, Furman NV, Malinova LI, Dolotovskaya PV. Monitoring of the effectiveness of antiplatelet therapy in cardiology practice. Rational Pharmacotherapy in Cardiology. 2017;13(1):107-15. (In Russ.) doi:10.20996/1819-6446-2017-13-1-107-115.
15. Mansurova JA, Zhunuspekova AS, Karazhanova LK. Reference values of platelet aggre¬gometry in healthy subjects. Klin Lab Diagn. 2018;63(9):549-52. (In Russ.).
16. Kim MH, Choi SY, An SY, Serebruany V. Validation of Three Platelet Function Tests for Bleeding Risk Stratification During Dual Antiplatelet Therapy Following Coronary Inter¬ventions. Clin Cardiol. 2016;39(7):385-90. doi:10.1002/clc.22540.
17. Pereira NL, Farkouh ME, So D, et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Se¬lection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA. 2020;324(8):761-71. doi:10.1001/jama.2020.12443.
About the Authors
A. A. KassymovaKazakhstan
Kassymova Assel A. — PhD candidate, Department of Cardiology and Interventional Arrhythmology
Semey
J. A. Mansurova
Kazakhstan
Mansurova Jamilya A. — Department of Cardiology and Interventional Arrhythmology
Semey
L. K. Karazhanova
Kazakhstan
Karazhanova Lyudmila K. — Doctor of medical science, Professor, Department of Cardiology and Interventional Arrhythmology
Semey
A. A. Chinybayeva
Kazakhstan
Chinybayeva Assel A. — PhD
Semey
Supplementary files
Review
For citations:
Kassymova A.A., Mansurova J.A., Karazhanova L.K., Chinybayeva A.A. Antiplatelet therapy de-escalation in a patient after percutaneous coronary intervention with a high risk of bleeding. Russian Journal of Cardiology. 2023;28(5):5274. https://doi.org/10.15829/1560-4071-2023-5274

















































