Scroll to:
The David procedure after the Ross operation: case series
https://doi.org/10.15829/1560-4071-2021-4767
Abstract
Pulmonary autograft in the aortic position provides high survival rate and quality of life for patients, low incidence of valve-related complications, and excellent hemodynamic characteristics both in the early and long-term period. However, in some patients in the long-term period, pulmonary autograft may dilate, which in turn is one of the reasons for reoperations. In patients who require surgery for annuloaortic ectasia with aortic valve (AV) regurgitation or aortic root aneurysm (or both) with normal AV leaflets, David procedure is considered.
Aim. To analyze results of David procedure after a prior Ross operation.
Material and methods. From April 2009 to December 2020, 212 Ross operations were performed on patients 18 years of age and older. The median age of the operated patients was 34 (27-45) years. In the long-term period, 10 (4,7%) patients required a second AV operation and 7 (3,3%) of them required another intervention on the ascending aorta due to aortic dilatation. Four out of 10 patients underwent David procedure. The follow-up period for patients after David procedure ranged from 2 to 84 months.
Results. The age of patients ranged from 23 to 45 years. Three patients had hypertension. The follow-up period from Ross's operation to David's one was 26 to 140 months. All patients had enlarged aortic annulus from 27 to 30 mm. The duration of myocardial ischemia ranged from 87 to 142 minutes, while the duration of artificial circulation — from 119 to 165 minutes. The graft diameter was 30 mm in two patients and 32 mm in the remaining ones. The length of stay in intensive care unit ranged from 16 to 23 hours. In the early postoperative period, no one had following postoperative complications: acute renal failure requiring hemodialysis, perioperative myocardial injury, stroke, sternal infection, respiratory failure requiring mechanical ventilation for ≥24 hours, resternotomy for bleeding and tamponade. In addition, there were no in hospital deaths. All patients had no aortic regurgitation at the time of discharge. All patients are alive and there were no reoperations. In one patient, in the long-term period, there was a mild aortic regurgitation, while in three patients — there is no regurgitation.
Conclusion. The presented case series show that David procedure can be performed safely and effectively in a significant number of patients requiring a second autograft surgery due to neosinus dilatation. In the medium term, the David procedure has shown good outcomes in these patients with 100% survival and no aortic regurgitation and reoperation.
Keywords
For citations:
Chernov I.I., Enginoev S.T., Kondrat'ev D.A., Zenkov A.A., Abdurakhmanov A.A., Tarasov D.G. The David procedure after the Ross operation: case series. Russian Journal of Cardiology. 2021;26(4S):4767. https://doi.org/10.15829/1560-4071-2021-4767
In patients with severe aortic valve (AV) pathology, its replacement is a method of surgical treatment [1][2]. Various types of prosthetic aortic valves have been proposed, including a pulmonary autograft (Ross operation) [3]. The pulmonary autograft in the aortic position provides high survival rate and quality of life, a minimal rate of valverelated complications, and excellent hemodynamic characteristics both in the early and long term [4-8]. However, in some patients in the long-term period after the Ross operation, pulmonary autograft dilatation may occur, which, in turn, is one of the reasons for resurgery [9-14]. In patients who require surgery for annuloaortic ectasia with AV regurgitation or aortic root aneurysm (or both) with normal AV leaflets, David procedure is considered [15]. In this article, we report four cases of David procedures being performed after the prior Ross operation.
Material and methods
Patients after the Ross operation. In our center, from April 2009 to December 2020, 212 Ross operations were performed on patients aged 18 years and older. The median age of patients was 34 (27-45) years. The main indication for surgery was severe aortic stenosis in 124 (58%) subjects, while severe aortic regurgitation in 88 (42%). Infective endocarditis was in 55 (26%) patients. Bicuspid AV was diagnosed in 131 (62%) patients. During the Ross operation, 14 (2,2%) patients underwent surgery on the ascending aorta. In all patients, pulmonary autograft was implanted using the full root replacement technique, and 54 (25%) patients underwent a modified Ross operation (Table 1).
Table 1
Initial demographic characteristics of patients at the time of the Ross operation
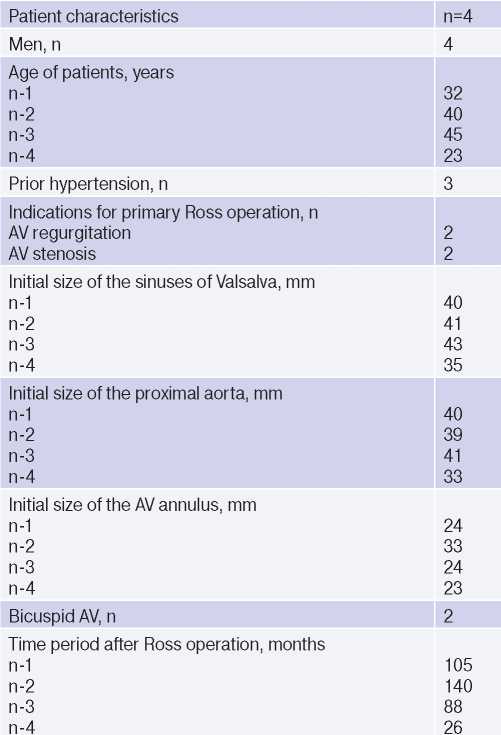
Abbreviation: AV — aortic valve.
Study population. In the long-term period, 10 (4,7%) patients required reoperation on the AV and 7 (3,3%) of them required another intervention on the ascending aorta due to aortic dilatation. Four out of 10 patients underwent David procedure. The age of patients was from 23 to 45 years. Three patients had prior hypertension. The follow-up duration from the Ross operation to the David procedure ranged from 26 to 140 months. All patients underwent transthoracic and transesophageal echocardiography (Figure 1), contrast-enhanced aortic computed tomography (Figure 2), while patients over 35 years of age underwent coronary angiography.
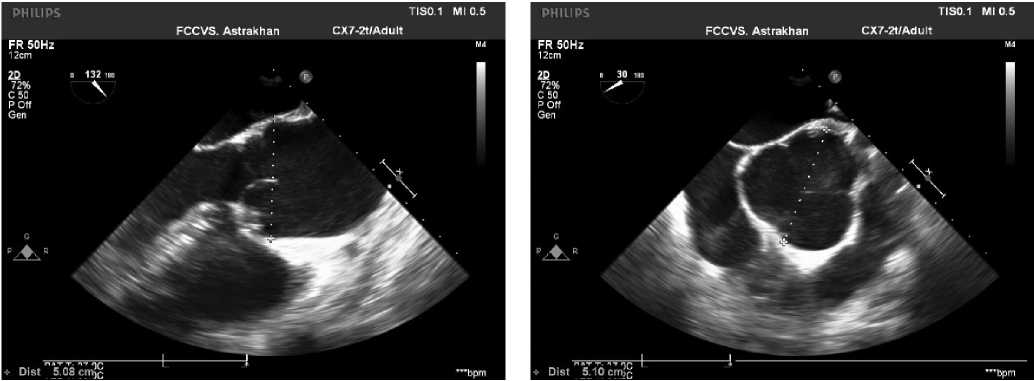
Figure 1. Intraoperative transesophageal echocardiography.
Note: dilatation of the sinuses of Valsalva and the proximal ascending aorta.
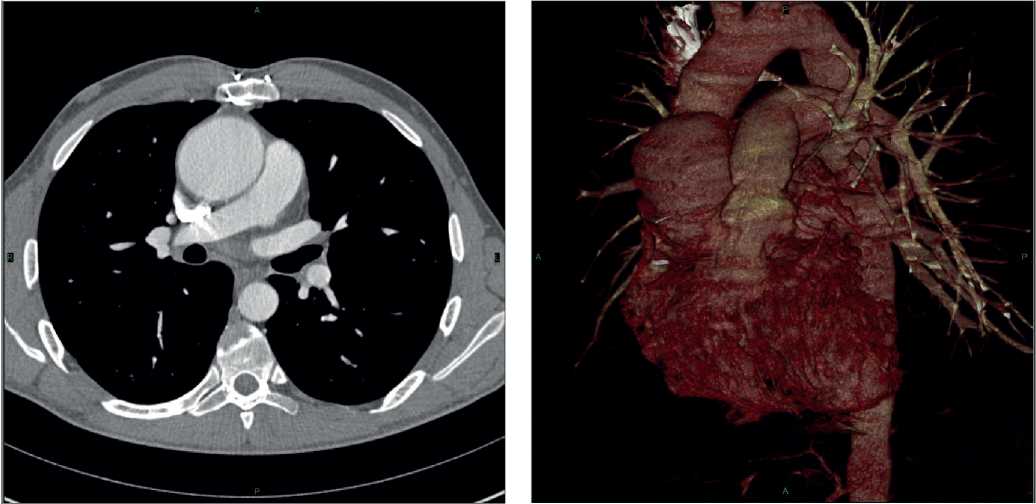
Figure 2. Contrast-enhanced aortic computed tomography.
Note: dilatation of the sinuses of Valsalva and the proximal ascending aorta.
Surgical technique. All reoperations were performed through median sternotomy. After cardiolysis, normothermic cardiopulmonary bypass was instituted. The left heart was drained through the right upper pulmonary vein. The aorta was cannulated as distally as possible, closer to the aortic arch. For cardioplegia, Custodiol at a dose of 2000 ml was used. Cardioplegic solution was delivered after transverse aortotomy, antegrade through the coronary artery orifices. The aortic root was maximally mobilized. From the left ventricular outflow tract, 9 to 12 sutures were placed (Ethibond 2/0”). A synthetic vascular prosthesis was implanted into the aortic root. The commissures and the remaining annulus part were sutured with a continuous suture using the Premilene 3/0-4/0 thread to the inner prosthesis wall. In the projection of the left and right coronary cusps, two holes were cut out on the prosthesis, and the coronary arteries orifices were implanted in them with a continuous suture (Premilene 6/0). The distal prosthesis anastomosis was formed with the ascending aorta in an end-to-end manner with a continuous suture (Premilene 4/0).
Results
All patients had AV annulus dilatation from 27 to 30 mm (Table 2). The duration of myocardial ischemia and cardiopulmonary bypass ranged from 87 to 142 minutes and from 119 to 165 minutes, respectively. The vascular prosthesis diameter was 30 mm in two patients and 32 mm in the remaining ones. The length of stay in the intensive care unit ranged from 16 to 23 hours (Tables 3-5). In the early postoperative period, none of the patients had following postoperative complications: acute renal failure requiring hemodialysis, perioperative myocardial injury, stroke, sternal infection, respiratory failure requiring mechanical ventilation ≥24 h, resternotomy due to bleeding and tamponade, and inhospital death. At the time of discharge, there were no aortic regurgitation in all patients. The follow-up period for patients after David’s operation ranged from 2 to 84 months. All patients are alive, and there were no reoperations. In one patient, a slight aortic regurgitation developed in the long-term period, while in three there was no regurgitation.
Table 2
Characteristics of patients at the time of the David procedure
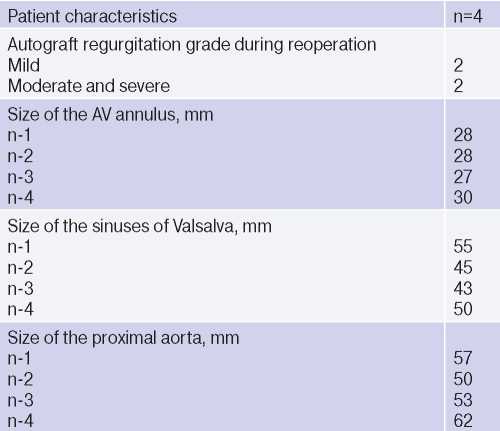
Table 3
Intraoperative parameters

Table 4
Postoperative parameters

Table 5
Postoperative echocardiographic parameters

Abbreviations: AV — aortic valve, AR — aortic regurgitation.
Discussion
The Ross operation has been proposed in AV surgery as a procedure that provides excellent hemodynamic parameters and freedom from anticoagulants [3][16][17]. One of its disadvantages is neoaortic root dilatation, which can lead to AV insufficiency and aortic wall dissection [14][18][19].
The main idea of this work was to show the potential of repeated valve-sparing surgery after the Ross operation in case of AV insufficiency and aortic dilation.
Isolated cases of David or Yacoub valve-sparing surgery after the Ross operation have been published in the literature [20-27]. Liebrich M, et al. published 18 cases of the David procedure after the Ross operation. The average period of reoperation was 11±3,2 years. The average age of operated patients was 49,8±13,9 years, mostly men (83%). There was no inhospital and 30-day mortality after the David procedure. During the follow-up period, one patient after 2,6 years required a second valve replacement surgery due to aortic regurgitation recurrence. Thus, the authors concluded that David procedure is possible after the Ross procedure with a low rate of mortality and postoperative complications [27].
In our opinion, one of the unresolved issues is the question of what neoaortic diameter should be operated on in such patients. In our practice, we focus on modern European guidelines for the diagnosis and treatment of aortic pathology [28]. Without significant aortic regurgitation, we perform surgical intervention with a diameter of 55 mm, while with significant aortic regurgitation — with a neoaortic diameter of 45 mm. A number of authors have described cases of Type A aortic dissection after the Ross operation [29][31]. Recently, Kalogerakos PD, et al. [32] concluded that aortic root dilatation is more unfavorable than mid-ascending aortic dilatation. According to this study, surgery should be considered when the diameter of aortic root and mid-ascending aorta is from 50 mm and 52,5 mm, respectively.
Several studies have confirmed that preoperative aortic regurgitation and morphological bicuspid AV are independent risk factors leading to neoaortic dilatation [16][20]. Based on this, as a prevention of aortic root dilatation, in our clinic, starting from 2014, all patients received modified Ross operation using a Dacron prosthetic vascular [21]. We also published the mid-term outcomes of this modification [33].
Conclusion
Progressive neosinus dilatation is one of the complications after Ross surgery. The presented case series show that David procedure can be performed safely and effectively in a significant number of patients requiring a second autograft surgery due to neosinus dilatation. In the medium term, the David procedure has shown good outcomes in these patients with 100% survival and no aortic regurgitation and reoperation.
Relationships and Activities: none.
References
1. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/ American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e35-e71. doi:10.1161/CIR.0000000000000932. Erratum in: Circulation. 2021;143(5):e228. Erratum in: Circulation. 2021;143(10):e784.
2. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739-91. doi:10.1093/eurheartj/ehx391.
3. Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet (London, England). 1967;2:956-8. doi:10.1016/s0140-6736(67)90794-5.
4. Chester AH, El-Hamamsy I, Butcher JT, et al. The living aortic valve: From molecules to function. Glob Cardiol Sci Pract. 2014;2014(1):52-77. doi:10.5339/gcsp.2014.11.
5. Bissell MM, Loudon M, Hess AT, et al. Differential flow improvements after valve replacements in bicuspid aortic valve disease: a cardiovascular magnetic resonance assessment. J Cardiovasc Magn Reson. 2018;20:10. doi:10.1186/s12968-018-0431-5.
6. David TE, David C, Woo A, et al. The Ross procedure: outcomes at 20 years. J Thorac Cardiovasc Surg. 2014;147:85-93. doi:10.1016/j.jtcvs.2013.08.007.
7. Martin E, Mohammadi S, Jacques F, et al. Clinical Outcomes Following the Ross Procedure in Adults: A 25-Year Longitudinal Study. J Am Coll Cardiol. 2017;70:1890-9. doi:10.1016/j.jacc.2017.08.030.
8. Mastrobuoni S, de Kerchove L, Solari S, et al. The Ross procedure in young adults: over 20 years of experience in our Institution. Eur J Cardio-Thoracic Surg. 2016;49:507-13. doi:10.1093/ejcts/ezv053.
9. Brown JW, Ruzmetov M, Rodefeld MD, et al. Incidence of and Risk Factors for Pulmonary Autograft Dilation After Ross Aortic Valve Replacement. Ann Thorac Surg. 2007;83:1781-9. doi:10.1016/j.athoracsur.2006.12.066.
10. Kouchoukos NT, Masetti P, Nickerson NJ, et al. The Ross procedure: long-term clinical and echocardiographic follow-up. Ann Thorac Surg. 2004;78:773-81. doi:10.1016/j.athoracsur.2004.02.033.
11. Elkins RC, Lane MM, McCue C, et al. Ross operation and aneurysm or dilation of the ascending aorta. Semin Thorac Cardiovasc Surg. 1999;11:50-4.
12. Simon-Kupilik N, Bialy J, Moidl R, et al. Dilatation of the autograft root after the Ross operation. Eur J Cardio-Thoracic Surg. 2002;21:470-3. doi:10.1016/S1010-7940(02)00016-7.
13. Luciani GB, Mazzucco A. Aortic root disease after the Ross procedure. Curr Opin Cardiol 2006;21:555-60. doi:10.1097/01.hco.0000245742.93453.1d.
14. David TE, Omran A, Ivanov J, et al. Dilation of the pulmonary autograft after the Ross procedure. J Thorac Cardiovasc Surg. 2000;119:210-20. doi:10.1016/S0022-5223(00)70175-9.
15. David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617-21; discussion 622.
16. Charitos EI, Hanke T, Stierle U, et al. Autograft reinforcement to preserve autograft function after the ross procedure: a report from the German-Dutch ross registry. Circulation. 2009;120:S146-54. doi:10.1161/CIRCULATIONAHA.108.843391.
17. Elkins RC, Thompson DM, Lane MM, et al. Ross operation: 16-year experience. J Thorac Cardiovasc Surg. 2008;136:623-30,630.e1-5. doi:10.1016/j.jtcvs.2008.02.080.
18. Hanke T, Stierle U, Boehm JO, et al. Autograft regurgitation and aortic root dimensions after the Ross procedure: the German Ross Registry experience. Circulation. 2007;116:I251-8. doi:10.1161/CIRCULATIONAHA.106.678797.
19. Luciani GB, Casali G, Favaro A, et al. Fate of the aortic root late after Ross operation. Circulation. 2003;108 Suppl:II61-7. doi:10.1161/01.cir.0000089183.92233.75.
20. Stelzer P. The Ross procedure: state of the art 2011. Semin Thorac Cardiovasc Surg. 2011;23:115-23. doi:10.1053/j.semtcvs.2011.07.003.
21. Chernov II, Kozmin DYu, Makeev SA, et al. Immediate results of the modified Ross operation. Pathology of blood circulation and cardiac surgery. 2016;20:12-8. (In Russ.)
22. Watanabe N, Saito S, Saito H, et al. Valve-sparing aortic root replacement with repair of leaflet prolapse after Ross operation. Interact Cardiovasc Thorac Surg. 2007;6:89-91. doi:10.1510/icvts.2006.137653.
23. Luciani GB, Viscardi F, Pilati M, et al. The Ross-Yacoub procedure for aneurysmal autograft roots: a strategy to preserve autologous pulmonary valves. J Thorac Cardiovasc Surg. 2010;139:536-42. doi:10.1016/j.jtcvs.2009.08.019.
24. Pettersson GB, Subramanian S, Flynn M, et al. Reoperations after the ross procedure in adults: towards autograft-sparing/Ross reversal. J Heart Valve Dis. 2011;20:425-32.
25. de Kerchove L, Boodhwani M, Etienne P-Y, et al. Preservation of the pulmonary autograft after failure of the Ross procedure. Eur J Cardio-Thoracic Surg Off J Eur Assoc Cardio-Thoracic Surg. 2010;38:326-32. doi:10.1016/j.ejcts.2010.02.014.
26. Luciani GB, Lucchese G, De Rita F, et al. Reparative surgery of the pulmonary autograft: experience with Ross reoperations. Eur J Cardio-Thoracic Surg Off J Eur Assoc Cardio-Thoracic Surg. 2012;41:1305-9. doi:10.1093/ejcts/ezr243.
27. Liebrich M, Weimar T, Tzanavaros I, et al. The David procedure for salvage of a failing autograft after the ross operation. Ann Thorac Surg. 2014;98:2046-52. doi:10.1016/j.athoracsur.2014.06.065.
28. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2014;35:2873-926. doi:10.1093/eurheartj/ehu281.
29. Richey S, Fiore AC, Huddleston CB. Type A Aortic Dissection After the Ross Procedure. Ann Thorac Surg. 2018;106:e105-6. doi:10.1016/j.athoracsur.2018.02.042.
30. Myers MR, Magruder JT, Crawford TC, et al. Surgical repair of aortic dissection 16 years post-Ross procedure. J Surg Case Reports. 2016;2016:rjw059. doi:10.1093/jscr/rjw059.
31. Siudalska H, Kusmierczyk M, Rozanski J, et al. Aortic dissection after the Ross procedure. Kardiol Pol. 2021:702-3. doi:10.33963/kp.15957.
32. Kalogerakos PD, Zafar MA, Li Y, et al. Root Dilatation Is More Malignant. Than. 2021:1-11. doi:10.1161/JAHA.120.020645.
33. Chernov II, Enginoev ST, Kondratyev DA, et al. Five-year outcomes of the modified Ross surgery in adults: experience from one center. Patologiya krovoobrashcheniya i kardiokhirurgiya = Circulation Pathology and Cardiac Surgery. 2021;25(3):43-50. (In Russ.) doi:10.21688/1681-3472-2021-3-43-50.
About the Authors
I. I. ChernovRussian Federation
Astrakhan.
Competing Interests:
None
S. T. Enginoev
Russian Federation
Astrakhan.
Competing Interests:
None
D. A. Kondrat'ev
Russian Federation
Astrakhan.
Competing Interests:
None
A. A. Zenkov
Russian Federation
Astrakhan.
Competing Interests:
None
A. A. Abdurakhmanov
Russian Federation
Astrakhan.
Competing Interests:
None
D. G. Tarasov
Russian Federation
Astrakhan.
Competing Interests:
None
Supplementary files
Review
For citations:
Chernov I.I., Enginoev S.T., Kondrat'ev D.A., Zenkov A.A., Abdurakhmanov A.A., Tarasov D.G. The David procedure after the Ross operation: case series. Russian Journal of Cardiology. 2021;26(4S):4767. https://doi.org/10.15829/1560-4071-2021-4767
JATS XML

















































