Scroll to:
Circadian rhythms of cardiac troponins: mechanisms and clinical significance
https://doi.org/10.15829/1560-4071-2020-4061
Abstract
Modern laboratory methods for determining biomarkers of cardiovascular diseases are highly sensitive and can detect almost single molecules in human biological fluids, significantly speeding up and improving the diagnosis of cardiovascular diseases. However, in this case, there is a decrease in specificity and it is necessary to take into account a number of additional factors that may affect the result of the study. Recent studies have shown that circadian rhythms (CR) are among these factors.
This review article is devoted to the discussion of recently discovered CR of cardiac troponins (CT). A number of articles reported that, both in healthy people and in patients with a number of chronic diseases, CT concentrations change during the day. Given that modern algorithms for diagnosing myocardial infarction (MI) are based on serial studies (0-1 h and 0-3 h) of blood serum, and the values of CT in the blood serum for the diagnosis of myocardial infarction (MI) for this period of time are only a few ng/l, the CT CR can to some extent affect the accuracy of MI diagnosis. Thus, natural physiological changes in the concentration of CT during the day can be mistakenly interpreted as diagnostically significant deviations and lead to an erroneous interpretation of laboratory test results.
For citations:
Chaulin A.M., Duplyakova P.D., Duplyakov D.V. Circadian rhythms of cardiac troponins: mechanisms and clinical significance. Russian Journal of Cardiology. 2020;25(3S):4061. https://doi.org/10.15829/1560-4071-2020-4061
The concept of circadian rhythms
Circadian rhythms (CR) are an evolutionary conservative mechanism that allows a wide range of organisms (from cyanobacteria to mammals) to adapt to cyclical changes in the external environment due to the Earth’s rotation on its axis (alternation of day and night) [12]. In a molecular level, CR consists of transcription-translation feedback loop of the central activators, genes, and proteins that are periodically expressed in almost every cell of the body. The circadian system is self-sufficient and averages a near-24-hour period [3].
CRs were discovered by the French astronomer and chronobiologist Jean-Jacques d’Ortous de Mairan (1729). The researcher noted that mimosa folded and unfolded leaflets regardless of light, in accordance with its 24-hour rhythm. In recent years, CRs have been actively studied and have been found in almost all earthlings. With the development of molecular genetic technologies, genes responsible for its regulation have been discovered. In the study by Benzer S and Konopka R on Drosophila, the authors identified a gene that regulates CRs and named it period (abbreviated as per). Mutations of this gene led to an impaired circadian clock in the experimental flies. Hall J, Rosbash M, and Young M made a huge contribution to the study of the molecular genetic mechanisms that underlie the CR, for which these researchers were awarded the Nobel Prize in Physiology or Medicine in 2017 [4][5][6][7].
CR are not only of great theoretical value, but also of great practical importance. It has been shown that an impaired circadian clock caused, for example, by shift (night) work, can lead to adverse health effects, namely, an increased risk of cancer, metabolic, cardiovascular, neurodegenerative and mental diseases [8][9][10][11].
CR are important in the laboratory diagnosis of some diseases due to the fact that some molecules are secreted at certain times of the day, for example, many hormones are more intensively secreted in the morning. In addition, a large number of molecules (metabolites) are influenced by these hormones, and therefore their concentrations also differ in the morning and evening. Therefore, for adequate diagnosis, clinicians should take into account the sampling time, and when a patient is hospitalized with an urgent medical condition, take into account possible physiological laboratory changes due to CR.
Recently, information has appeared on the CR of cardiac troponins (cTn) — key biomarkers of a number of cardiovascular diseases (CVD), in particular, myocardial infarction (MI). In this review, we will analyze the studies published to date on the CR of cTn and discuss their possible mechanisms. There are currently no such papers.
Biological basis of cTn
For a long time, cTn was considered strongly intracellular molecules, and its detection in blood serum was considered one of the MI criteria [12][13]. The significantly increased sensitivity of new methods for cTn assay (high-sensitivity and ultrasensitive) contributed to changes in the understanding of their biology and diagnostic value. CTn molecules in serum circulate as a heterogeneous pool: free single cTn molecules, binary- and ternary-complexed forms. The immunochemical test kit for cTn assay includes antibodies to different antigenic determinants of the cTn molecule, which determines the difference in values, as a result of which laboratory parameters of the same patient may differ several times [14].
In the cardiomyocyte, cTn molecules are located as a structural pool (troponin complex) and a cytosolic (free) pool. Free cTn molecules are the main contributors to an increase in cTn concentration in healthy patients and in reversible/minor myocardial damage during the urgent events, as well as during exercise or psychological stress. In addition, the cytosolic pool is the first to be released during the MI, forming the first peak, and the second peak in serum cTn occurs when the structural pool is degraded.
CTn molecules are present both in serum/plasma and in a number of other biological fluids resulting from filtration and secretion processes, in particular, in urine and oral fluid [15][16][17][18]. At the same time, cTN molecules are relatively large in relation to the glomerular capillary pores and are found in urine not in all patients [19]. The probability of detecting cTn in urine depends on the sensitivity of the test method used.
Circadian rhythms of cTn
Clinical trials were searched for in the PubMed database for the following keywords: circadian rhythm, diurnal rhythm, cardiac troponin, high-sen- sitivity troponin. A total of 10 original studies were found that examined CR of cTn. Wu A and Vasile V were the first to report them [20][21][22].
Aakre K, et al. studied weekly and 90-minute biological variations in the concentration of cTnT and cTnI using high-sensitivity methods — Roche Diagnostics and Abbott Diagnostics, respectively [23]. Blood samples were drawn from healthy people and patients with chronic kidney disease (CKD) at 90-minute intervals over a 6-hour period (8:30 am to 2:30 pm). It turned out that the cTnT (in healthy controls and patients with CKD) and cTnI (only in the group of CKD patients) levels decreased hourly by an average of 0,8-1,7%. Weekly differences in cTnT and cTnI levels were 8 and 15%, respectively [23].
In several studies, CR of cTn was studied in more detail — the measurement of the cTn level in the same patients was carried out every hour during the day, which makes it possible to establish the CR much more accurately [24][25][26][27]. The clinical characteristics of the patients participating in these studies varied significantly (Table 1).
Table 1
Studies on CRs of cTn
|
№ |
The number of patients participating in the study and their baseline characteristics |
Study design |
Key findings |
Source |
|---|---|---|---|---|
|
1 |
Healthy (n=12) |
4-hour hourly cTnI determination |
Population-specific reference ranges for cTnI are less accurate than individual patient-specific |
[20] |
|
2 |
Healthy (n=20) |
Determination of cTnT at 5 time points |
cTnT showed greater diurnal variability compared to cTnI |
[21] |
|
3 |
Healthy (n=20) |
Determination of cTnT at the study beginning and after 1, 2, 3, and 4 hours |
Circadian variability in cTnT levels is insignificant, but maybe significant in the analysis. |
[22] |
|
4 |
Healthy (n=24) |
Determination of cTnT and cTnI was carried out hourly for 25 hours (from 8:30 to 8:30) |
cTnT is characterized by CR with a peak morning concentration and a gradual decrease during the day. For cTnI, circadian concentration changes are insignificant (no more than 1 ng/L) |
[23] |
|
5 |
Healthy (n=20) Patients with CKD (dialysis) (n=19) |
Determination of cTnT and cTnI was carried out at 90-minute intervals for 6 hours (8:30-14:30) |
For cTnT and cTnI, CR has a gradual decrease in concentration during the day by 0,8-1,7% hourly |
[24] |
|
6 |
Patients with type 2 diabetes without acute CVD (n=23) Patients with type 2 diabetes without acute CVD (n=7) |
Determination of cTnT in serial venous blood samples, which was collected over an 11-hour period (from 8:30 to 19:30) Determination of cTnT in serial blood samples, which was taken hourly for 25 hours (from 8:30 am to 8:30 am the next day) |
cTnT is characterized by CR with a peak concentration in the morning and a gradual decrease in concentration during the day. |
[25] |
|
7 |
Patient with CRF |
Hourly determination of cTnT and cTnI in serum using high-sensitivity methods |
cTnT is characterized by statistically and clinically significant CRs, which, among other things, can affect the accuracy of MI diagnosis when using rapid algorithms |
[26] |
|
8 |
Healthy (n=17) |
To study the CR of cTn among healthy individuals during the day (from 08:00 to 08:00) |
The maximum values of cTnT were recorded at 6:00, and the minimum at 18:00 |
[27] |
|
9 |
Patients admitted to the emergency department with suspected MI (n=2601) |
Determination of cTnI using four methods of analysis. Morning and evening values of cTnI were compared in the same patients |
Circadian cTnI variations are statistically and clinically insignificant |
[29] |
|
10 |
Healthy (n=35) |
Determination of cTn was carried out using a high-sensitivity method in blood samples obtained in the morning (8:009:00) and after 1, 2, 3, and 7 hours) |
For cTn, the diurnal concentration rhythm is characteristic. The highest concentrations were in the morning, and decreased in the evening. |
[30] |
Abbreviations: MI — myocardial infarction, CVD — cardiovascular diseases, cTn — cardiac troponins, cTnT — cardiac troponin T, cTnI — cardiac troponin I, CKD — chronic kidney disease, CRF — chronic renal failure, CR — circadian rhythms.
Fournier S, et al. studied circadian changes in cTnT using the cosinor model in 17 healthy individuals (mean age, 25 years) [27]. Participants followed a specific day regimen three days before the study — a specially designed diet with meals at 8:30, 12:00, and 19:00, physical activity in the form of 10-minute walking before and after meals. Participants were asked to go to bed at 21:00 and turn off the lights at 22:00. The quality of their CR was assessed by measuring body temperature, heart rate, and blood pressure before each blood draw. Blood sampling was carried out every 4 hours during the day. The concentration of cTnT during the day ranged from 3 to 9 ng/L, while values above the 99th percentile (14 ng/L) were not observed. The concentration peaked at 06:00, and the maximum decrease was observed at 18:00. This study demonstrated the CR of cTnT concentration with an amplitude of 20,5%.
Klinkenberg L, et al. studied CR of cTn in patients with type 2 diabetes [24], as well as in healthy individuals [25]. Comparing both of these studies, it can be noted that in patients with type 2 diabetes, circadian changes in cTnT levels were slightly higher than in healthy controls. The authors concluded that circadian changes in cTnT levels may affect the results if cTnT is studied in screening programs, but they are unlikely to affect the accuracy of MI diagnosis using guidelines of the European Society of Cardiology.
CRT in patients with and without CKD were studied by van der Linden N, et al. [28]. The authors performed hourly changes in cTnI concentration in 36 subjects, half of whom had CKD. Baseline cTnI concentrations were insignificantly higher in the CKD group compared with the non-CKD group (median [IQR] 6,8 [3,5-9,2] ng/L vs 4,7 [2,8-6,9] ng/L; р=0,09).
In all the studies mentioned above, CR were present in cTnT, but were not significant for cTnI [25][26][27]. Wildi K, et al. also did not find CR of cTnI characteristic of cTnT [29]. Remarkably, in the Klinkenberg L study, the hourly differences in cTnI concentration were no more than 1 ng/L [25], while in the patient with CKD in the van der Linden N study, it reached 2,6 ng/L [26]. Comparing the results of the data from Klinkenberg L and van der Linden N [25][26], we also note that in the latter study, CR of cTnT were much more significant and could affect the accuracy of MI diagnosis (when using early diagnostic algorithms — 0-1 h, and 0-3 h). In this regard, it can be assumed that circadian changes in the concentrations of both cTnT and cTnI in patients with CKD are much more pronounced than in healthy individuals.
In 2020 Zaninotto M, et al. even when using a high-sensitivity assay, the CR of cTnI concentration was found [30]. It is noteworthy that the CRs of cTn correspond to the CRs of another biomarker of MI, creatine phosphokinase-MB (CPK-MB) [31].
There is another (surrogate) approach to studying CRs. Its purpose is to show whether clinically significant daily changes in cTnT concentration are present in patients in actual clinical practice. Ross A, et al. analyzed patients admitted to the emergency department with chest pain but did not have MI or other conditions that could affect blood cTnT levels [32]. The time periods for blood sampling were as follows: 00:00-03:59, 04:00-07:59, 08:00-11:59, 12:00-15:59, 16:00-19:59 and 20:00-23:59. A total of 19460 patients (men, 50%) were included in the study (mean age, 54+16 years). Patients who were admitted at night were younger, but similar in other characteristics. The highest mean values of cTnT concentration at admission were in men (9,0 ng/L; 95% confidence interval (CI), 8,7-9,3) and in women (8,0 ng/L; 95% CI, 7,8-8,2) were detected between 08:00-11:59. After adjusting for age and estimated glomerular filtration rate (GFR), no significant CR changes in cTnT concentration were found.
An interesting idea is to compare the time of MI onset and its size with CRs of cTn. Suarez-Barrientos A, et al. retrospectively analyzed the data of 811 patients with ST-segment elevation MI (STEMI), evaluating the size of MI by the peak concentration of CPK-MB and cTnI [33]. The authors compared their values in four-time intervals: 24:00-06:00; 06:00-12:00; 12:00-18:00; 18:00-24:00. The largest number of patients with MI was admitted between 06:00 and 12:00 (n=269). Patients with myocardial infarction of the anterior LV wall were more often hospitalized between 24:00 and 12:00. Primary percutaneous coronary intervention (PCI) was performed somewhat more frequently in patients admitted between 24:00 and 06:00 (86% of patients). The peak concentration for both enzymes was observed from 06:00 to 12:00, and the minimum — from 12:00 to 18:00. The infarct size was significantly larger in patients hospitalized during the period of 06:0012:00 than in patients with the onset of STEMI at any other time (CFK-MB: 1766,84 U/L (95% CI, 1554,34-2020,69) vs 1517,21 U/L (95% CI, 1382,411649,81); cTnI: 66,65 ng/ml (95% CI, 56,47- 78,76) vs 53,65 ng/ml (95% CI, 47,7-60,04).
The rationale of routine thrombus aspiration during PCI is currently questionable. Meanwhile, according to the registry of Acute Myocardial Infarction in Switzerland Plus, which included 3648 patients with STEMI and primary PCI in 2009-2014 (aspiration was performed in 49% of patients), the efficiency of aspiration was most effective in reducing the infarct size in the period from 06:00 to 17:59 [34].
Interestingly, CRs were also found during the type 4a MI — in patients undergoing elective PCI. Fournier S, et al. analyzed the data of 1021 patients depending on the PCI time: the morning group (n=651) — 07:00-12:00; the daytime group (n=370) — 12:00-19:00. The incidence of peripro- cedural MI was significantly lower in the morning group compared with the daytime group (20% vs 30%, p<0,001). This difference remained significant after sex and age adjustment (21% vs 29%, p=0,03). According to multivariate analysis, PCI in the afternoon independently increases the risk of periproce- dural MI (odds ratio, 2,0; 95% CI, 1,1-3,4; p=0,02) [35].
Possible mechanisms of cTn CRs
Recent studies have shown that cTn molecules can be released from cardiomyocytes in healthy individuals; however, the mechanisms of this release are still not completely known [14][18][36]. Recent studies have shown that even minor changes in the activity of various body systems, in particular, such as the neuroendocrine, hemostatic, and urinary systems, can affect the serum cTn level. Since these systems have their own CRs, they can presumably cause CRs of cTn.
Role of the neuroendocrine system in cTn release. The neuroendocrine system is vital for adequate cardiovascular functioning. Lazzarino A, et al. found that stress-related cortisol release is associated with cTnT levels [37]. Minor physical activity and administration of dobutamine, according to Samaha E, et al., also led to a slight increase in serum cTn levels [38]. Catecholamines are known to increase myocardial oxygen demand and heart rate (HR). Ben Yedder N, et al. found a close correlation with the cTnT level (r=0,637, p<0,01). In addition, in cTnT- positive patients, HR during paroxysm was significantly higher than in troponin-negative patients (190 bpm vs 170 bpm; p=0,008) [39].
The mechanism of the cTn release in sharp HR increase and tachyarrhythmias is presumably associated with an imbalance between the demand and delivery of oxygen and metabolic substrates (glucose, free fatty acids, lactate, etc.). In conditions of increased HR, the myocardium suffers from energy deficit and cannot fully ensure the constancy of the intracellular environment. Oxygen deficiency leads to a switch of metabolic pathways from aerobic to anaerobic, gradual accumulation of lactate and intracellular acidification, in which protease enzymes are activated that break down intracellular proteins, including those that are part of biological membranes, which ultimately leads to permeability increase and release of cTn molecules [14][18][40][41]. The size of cTn molecules allows them to pass through the relatively intact cell membranes of car- diomyocytes. In addition, it is known that the cytoplasmic (free) pool of cTn is located in the cytosol of cardiomyocytes, which, according to some researchers, is released in healthy individuals, as well as in case of minor (reversible) damage to cardiomyocytes [14][18][42] (Figure 1).
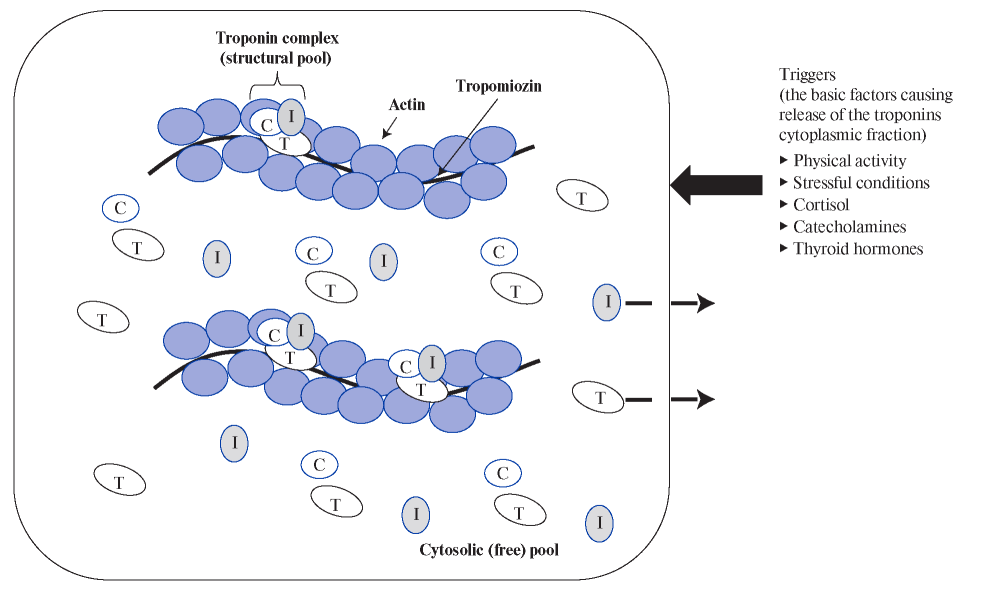
Figure 1. Localization of cTn molecules in cardiomyocytes (cytosolic and structural pool) and the main triggers causing the release
of the cytoplasmic fraction.
Another trigger for the cTn release from the myocardium may be thyroid hormones (T3, T4). The activity of thyrotropic cells of the anterior pituitary gland increases in the morning, which leads to an increase in the level of thyroid-stimulating hormone, which, in turn, activates the thyroid follicular cells to produce T3 and T4. It was noted that the morning serum concentration of T3 and T4 exceeds the evening values [43][44]. At the same time, higher levels of thyroid hormones in the morning are associated with a more severe clinical performance of CVD (coronary artery disease, arterial hypertension, and atrial fibrillation) in these patients [43][44]. An increase in the level of thyroid hormones leads to HR increase, and, as a result, an imbalance between the demand and delivery of oxygen, which, most likely, is the cause of cTn CRs (higher morning concentration of cTn).
Role of the hemostatic system. Along with changes in the activity and functioning of the neuroendocrine system, circadian changes in the activity of the hemostatic system may also be responsible for cTn release. The activity of hemostasis changes during the day: for example, in the morning, as a rule, procoagulant activity prevails, contributing to a change in the blood rheological properties towards the viscosity increase [45][46]. It is well known that there is a link between high platelet aggregation capacity in the morning and an increased risk of MI and sudden death during this period [46].
In addition, taking into account the role of hemostatic enzymes, in particular, thrombin, in the specific cTnT cleavage into two fragments [47][48], and, accordingly, the change in the availability of antigenic determinants for diagnostic antibodies, it can be suggested that this mechanism is involved in the formation of CRs for some test systems.
Role of cTn biochemical differences in the CR formation. For cTnI, unlike cTnT, CRs are practically not characteristic [28][29]. However, the reason for this has not been finally established. These possible differences can be explained by the biochemical characteristics of cTnT and cTnI molecules (Table 2).
Table 2
Biochemical and laboratory features of cTn molecules
|
Distinctive characteristics |
cTnT |
cTnI |
|---|---|---|
|
Specificity for detecting cardiomyocyte damage |
Almost absolute (there are several studies where the cTnT expression was found in skeletal muscle and the walls of the pulmonary veins and vena cava) |
Absolute (only myocardial expression) |
|
Molecular mass |
36 kDa |
23 kDa |
|
Total cTn content in mg per 1 g of myocardial tissue weight |
10-11 mg/g of myocardium |
4-6 mg/g of myocardium |
|
cTn cytosolic pool volume |
6-7% |
3-4% |
|
Laboratory diagnostics |
Practically standardized (there is only one manufacturer of a high-sensitivity test for determining cTn) |
Non-standardized (there are a huge number of manufacturers of diagnostic tests, which give different results in the same patient) |
Abbreviations: cTn — cardiac troponins, cTnT — cardiac troponin T, cTnI — cardiac troponin I.
Along with numerous and important similarities between cTnI and cTnT molecules, including high myocardial specificity, high diagnostic accuracy and correlation with the severity of myocardial damage, and prognostic value in MI, there are a number of very important differences. The molecular weight and, accordingly, the size of cTnI molecule is smaller, and therefore this protein is much easier to pass through the cell membrane of cardiomyocytes. And the total cTnT content in the myocardium and the volume of cytoplasmic (free) cTnT is almost two times higher than cTnT (10-11 mg/g vs 4-6 mg/g and 6-7% vs 3- 4%, respectively) [29]. Taking into account that the content of cTnT in the myocardium and the volume of the cytoplasmic fraction are higher, it can be assumed that the possibility of releasing cTnT is much higher, which may to some extent explain the CR differences.
Differences in the ability to cross the glomerular filtration barrier may also explain why more pronounced circadian changes are characteristic of cTnT. Several studies have shown that in patients with CKD, the level of cTnT increases more often and reaches higher values compared with cTnI [49].
Role of the urinary system in formation of cTn CRs and cTn level increase. As previously reported, the serum concentration of cTn depends not only on their release from the myocardium, but also on the elimination. Comparison of Klinkenberg L, et al. [24][25] and van der Linden N, et al [26] studies show that in patients with normal renal function, less pronounced circadian changes in cTnI were observed than in patients with depressed renal function. This trend is also characteristic of cTnT: in patients with adequate renal filtration (normal GFR), circadian changes are much more pronounced.
The urinary system also changes its activity during the day. It is known that morning urine output is higher than nighttime, which indicates a higher GFR in the morning [50]. It is noteworthy that the factors causing an increase in GFR also cause an increase in cTn clearance — patients with higher blood pressure have higher elimination of cTn [16]. Consequently, CRs of the urinary system and factors affecting GFR may also be associated with circadian variations in serum cTn concentration.
Renal function may play a significant role in the difference between the CRs of cTnT and cTnI due to the fact that a GFR decrease is much more closely associated with a cTnT increase [49].
Conclusion
The available publications indicate the presence of cTn CRs, which are more pronounced in cTnT. CTnI level probably has no circadian variation or is insignificant. Diurnal cTn changes, apparently, can affect the diagnosis, and more importantly, the risk stratification in some emergency conditions, when the cTn level increases insignificantly or moderately. At the same time, the CRs of cTn is unlikely to have a significant impact on the accuracy of MI diagnosis. It has to be considered that most of the patients with MI belong to the older age group and have many comorbidities (eg, CKD). In these cases, the clinical significance of cTn CRs may be significant.
However, at the present time we are just beginning to study the cTn CRs, as well as the factors influencing them. Therefore, final conclusions can be drawn only after conducting large-scale studies.
References
1. Ouyang Y, Andersson CR, Kondo T, et al. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95(15):8660-8664. doi:10.1073/pnas.95.15.8660
2. Hut RA, Beersma DG. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philos Trans R Soc Lond B Biol Sci. 2011;366(1574):2141-2154. doi:10.1098/rstb.2010.0409
3. Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177-2181. DOI: 10.1126/science.284.5423.2177
4. Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68(9):2112-2116. doi:10.1073/pnas.68.9.2112
5. Hall JC, Rosbash M. Genetic and molecular analysis of biological rhythms. J Biol Rhythms. 1987;2(3):153-178. doi:10.1177/074873048700200301
6. Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312(5996):752-754. doi:10.1038/312752a0
7. URL: https://www.nobelprize.org/prizes/medicine/2017/advanced-information/
8. Hedström AK, Åkerstedt T, Hillert J, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol. 2011;70(5):733-741. doi:10.1002/ana.22597
9. Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi:10.1136/bmj.i5210
10. Wyse CA, Celis Morales CA, Graham N, et al. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann Med. 2017;49(5):411-420. DOI: 10.1080/07853890.2017.1292045
11. Stenvers DJ, Scheer FAJL, Schrauwen P, et al. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75-89. doi:10.1038/s41574-018-0122-1
12. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21(18):1502‐1513. doi:10.1053/euhj.2000.2305.
13. Ladenson JH. A personal history of markers of myocyte injury [myocardial infarction]. Clin Chim Acta. 2007;381(1):3-8. doi:10.1016/j.cca.2007.02.039
14. Chaulin AM, Karslyan LS, Grigorieva EV, et al. Metabolism of cardiac troponins (literature review). Complex Issues of Cardiovascular Diseases. 2019;8(4):103-115. In Russian. Чаулин А.М., Карслян Л.С., Григорьева Е.В., и др. Особенности метаболизма сердечных тропонинов (обзор литературы). Комплексные проблемы сердечно-сосудистых заболеваний. 2019;8(4):103-115. DOI: 10.17802/2306-1278-2019-8-4-103-115
15. Garcia-Osuna A, Gaze D, Grau-Agramunt M, et al. Ultrasensitive quantification of cardiac troponin I by a Single Molecule Counting method: analytical validation and biological features. Clin Chim Acta. 2018;486:224-231. doi:10.1016/j.cca.2018.08.015
16. Pervan P, Svaguša T, Prkačin I, et al. Urine high sensitive Troponin I measuring in patients with hypertension. Signa Vitae - A Journal In Intensive Care And Emergency Medicine. 2017;13(Suppl 3):62-64. https://doi.org/10.22514/SV133.062017.13
17. Mirzaii-Dizgah I, Riahi E. Salivary high-sensitivity cardiac troponin T levels in patients with acute myocardial infarction. Oral Dis. 2013;19(2):180-184. doi:10.1111/j.1601-0825.2012.01968.x
18. Chaulin AM, Karslyan LS, Bazyuk EV, et al. Clinical and Diagnostic Value of Cardiac Markers in Human Biological Fluids. Kardiologiia. 2019;59(11):66-75. In Russian. Чаулин А.М., Карслян Л.С., Григорьева Е.В., и др. Клинико-диагностическая ценность кардиомаркеров в биологических жидкостях человека. Кардиология. 2019;59(11):66-75. DOI:10.18087/cardio.2019.11.n414
19. Ziebig R, Lun A, Hocher B, et al. Renal elimination of troponin T and troponin I. Clin Chem. 2003;49(7):1191-1193. DOI: 10.1373/49.7.1191
20. Wu AH, Lu QA, Todd J, et al. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52-58. doi:10.1373/clinchem.2008.107391
21. Vasile VC, Saenger AK, Kroning JM, et al. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(7):1086-1090. doi:10.1373/clinchem.2009.140616
22. Vasile VC, Saenger AK, Kroning JM, et al. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(7):1086-1090. doi:10.1373/clinchem.2009.140616
23. Frankenstein L, Wu AH, Hallermayer K, et al. Biological variation and reference change value of high-sensitivity troponin T in healthy individuals during short and intermediate follow-up periods. Clin Chem. 2011;57(7):1068-1071. doi:10.1373/clinchem.2010.158964
24. Aakre KM, Røraas T, Petersen PH, et al. Weekly and 90-minute biological variations in cardiac troponin T and cardiac troponin I in hemodialysis patients and healthy controls. Clin Chem. 2014;60(6):838-847. doi:10.1373/clinchem.2013.216978.
25. Klinkenberg LJ, van Dijk JW, Tan FE, et al. Circulating cardiac troponin T exhibits a diurnal rhythm. J Am Coll Cardiol. 2014;63(17):1788-1795. doi:10.1016/j.jacc.2014.01.040
26. Klinkenberg LJ, Wildi K, van der Linden N, et al. Diurnal Rhythm of Cardiac Troponin: Consequences for the Diagnosis of Acute Myocardial Infarction. Clin Chem. 2016;62(12):1602-1611. doi:10.1373/clinchem.2016.257485.
27. van der Linden N, Cornelis T, Klinkenberg LJ, et al. Strong diurnal rhythm of troponin T, but not troponin I, in a patient with renal dysfunction. Int J Cardiol. 2016;221:287-288. doi:10.1016/j.ijcard.2016.06.268
28. Fournier S, Iten L, Marques-Vidal P, et al. Circadian rhythm of blood cardiac troponin T concentration. Clin Res Cardiol. 2017;106(12):1026-1032. doi: 10.1007/s00392-017-1152-8.
29. van der Linden N, Hilderink JM, et al. Twenty-Four-Hour Biological Variation Profiles of Cardiac Troponin I in Individuals with or without Chronic Kidney Disease. Clin Chem. 2017;63(10):1655-1656. doi:10.1373/clinchem.2017.275107
30. Wildi K, Singeisen H, Twerenbold R, et al. Circadian rhythm of cardiac troponin I and its clinical impact on the diagnostic accuracy for acute myocardial infarction. Int J Cardiol. 2018;270:14-20. doi:10.1016/j.ijcard.2018.05.136
31. Zaninotto M, Padoan A, Mion MM, et al. Short-term biological variation and diurnal rhythm of cardiac troponin I (Access hs-TnI) in healthy subjects. Clin Chim Acta. 2020;504:163-167. doi:10.1016/j.cca.2020.02.004
32. Gutenbrunner C. Circadian variations of the serum creatine kinase level--a masking effect? Chronobiol Int. 2000;17(4):583-590. doi:10.1081/cbi-100101065.
33. Roos A, Holzmann MJ. Diurnal variation in admission troponin concentrations in patients with chest pain in the emergency department. Clin Biochem. 2018;54:18-24. doi: 10.1016/j.clinbiochem.2018.02.003.
34. Suárez-Barrientos A, López-Romero P, Vivas D, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97(12):970-976. doi: 10.1136/hrt.2010.212621.
35. Fournier S, Muller O, Benedetto U, et al.; on behalf on the AMIS Plus Investigators. Circadian dependence of manual thrombus aspiration benefit in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clin Res Cardiol. 2018;107(4):338-346. doi: 10.1007/s00392-017-1189-8.
36. Fournier S, Puricel S, Morawiec B, et al. Relationship between time of day and periprocedural myocardial infarction after elective angioplasty. Chronobiol Int. 2014;31(2):206-213. doi: 10.3109/07420528.2013.839561.
37. de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA. 2013;309(21):2262-2269. doi:10.1001/jama.2013.5809
38. Lazzarino AI, Hamer M, Gaze D, et al. The association between cortisol response to mental stress and high sensitivity cardiac troponin T plasma concentration in healthy adults. J Am Coll Cardiol. 2013;62(18):1694-1701. doi: 10.1016/j.jacc.2013.05.070.
39. Samaha E, Brown J, Brown F, et al. High-sensitive cardiac troponin T increases after stress echocardiography. Clin. Biochem. 2019;63:18-23. URL: https://doi.org/10.1016/j. clinbiochem.2018.11.013
40. Ben Yedder N, Roux JF, Paredes FA. Troponin elevation in supraventricular tachycardia: primary dependence on heart rate. Can. J. Cardiol. 2011;27(1):105-109. doi: 10.1016/j. cjca.2010.12.004.
41. Chaulin AM, Duplyakov DV. Increased cardiac troponins, not associated with acute coronary syndrome. Part 2. Kardiologiya: novosti, mneniya, obuchenie [Cardiology: News, Opinions, Training]. 2019;7(2):24-35. (in Russian) Чаулин А.М., Дупляков Д.В. Повышение кардиальных тропонинов, не ассоциированное с острым коронарным синдромом. Часть 2. Кардиология: новости, мнения, обучение. 2019;7(2):24–35. doi: 10.24411/2309-1908-2019-12003.
42. Nelson D., Cox M. Lehninger Principles of Biochemistry. Vol. 2. Bioenergetics and metabolism. М.: Binom; 2011. 694 p. (in Russian). Нельсон Д., Кокс М. Основы биохимии Ленинджера. Т. 2. Биоэнергетика и метаболизм. М.: Бином; 2011. 694 с.
43. Hessel MHM, Atsma DE, van der Valk EJM, et al. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Eur J Physiol. 2008;455(4):979-986. doi:10.1007/ s00424-007-0354-8
44. Tsareva YuO, Mayskova EA, Fedotov EA, et al. Circadian rhythms of thyroid hormones in patients with ischemic heart disease, arterial hypertension, and atrial fibrillation. Kardiologiia. 2019;59(3S):23-29. In Russian. Царева Ю.О., Майскова Е.А., Федотов Э.А., и др. Циркадные ритмы тиреоидных гормонов у пациентов с ишемической болезнью сердца, артериальной гипертонией и фибрилляцией предсердий. Кардиология. 2019;59(3S):23-29. DOI: 10.18087/cardio.2506.
45. Tsareva YuO, Sokolov IM, Aristarin MA. Thyroid function and its biorhythmic changes in coronary heart disease and atrial fibrillation. Modern problems of science and education. 2015;1-1. In Russian. Царева Ю.О., Соколов И.М., Аристарин М.А. Функция щитовидной железы и ее биоритмические изменения при ишемической болезни сердца и фибрилляции предсердий. Современные проблемы науки и образования. 2015;1-1.; URL: http://science-education.ru/ru/article/view?id=18254
46. Andrews NP, Gralnick HR, Merryman P, et al. Mechanisms underlying the morning increase in platelet aggregation: a flow cytometry study. J Am Coll Cardiol. 1996;28(7):1789-1795. doi:10.1016/S0735-1097(96)00398-1
47. Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316(24):1514-1518. doi:10.1056/NEJM198706113162405
48. Streng AS, de Boer D, van Doorn WP, et al. Identification and Characterization of Cardiac Troponin T Fragments in Serum of Patients Suffering from Acute Myocardial Infarction. Clin Chem. 2017;63(2):563-572. doi:10.1373/clinchem.2016.261511
49. Katrukha IA, Kogan AE, Vylegzhanina AV, et al. ThrombinMediated Degradation of Human Cardiac Troponin T. Clinical Chemistry. 2017;63(6):1094-1100. https://doi.org/10.1373/clinchem.2016.266635
50. Rubini Gimenez M, Twerenbold R, Reichlin T, et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J. 2014;35(34):2303-2311. doi:10.1093/eurheartj/ehu188
51. Bryukhanov V.M., Zvereva A.J. The kidney role in regulation of circade rithms of the organism. Nephrology. 2010;14(3):17-31. In Russian. Брюханов В.М., Зверева А.Я. Роль почки в регуляции суточных ритмов организма. Нефрология. 2010;14(3):17-31. https://elibrary.ru/item.asp?id=15217177
About the Authors
A. M. ChaulinRussian Federation
Samara
P. D. Duplyakova
Russian Federation
Samara
D. V. Duplyakov
Russian Federation
Samara
Supplementary files

|
1. Титульный лист и доп информация | |
| Subject | ||
| Type | Исследовательские инструменты | |
Download
(16KB)
|
Indexing metadata ▾ | |
|
|
2. Рисунок для статьи | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(155KB)
|
Indexing metadata ▾ | |
Review
For citations:
Chaulin A.M., Duplyakova P.D., Duplyakov D.V. Circadian rhythms of cardiac troponins: mechanisms and clinical significance. Russian Journal of Cardiology. 2020;25(3S):4061. https://doi.org/10.15829/1560-4071-2020-4061
JATS XML

















































